Sonographic evaluation of diaphragmatic thickness and excursion as a predictor for successful extubation in mechanically ventilated preterm infants
- Original Article
- Published: 28 September 2020
- Volume 180 , pages 899–908, ( 2021 )
Cite this article

- Eslam Bahgat 1 ,
- Hanan El-Halaby 2 ,
- Ashraf Abdelrahman 3 ,
- Nehad Nasef ORCID: orcid.org/0000-0001-7650-123X 1 , 2 , 4 &
- Hesham Abdel-Hady 1 , 2
3238 Accesses
18 Citations
7 Altmetric
Explore all metrics
A Correspondence to this article was published on 12 November 2020
Sonographic assessment of diaphragmatic thickness and excursion has been found to be an accurate tool in predicting successful extubation of adult patients from invasive mechanical ventilation. We aimed to evaluate the accuracy of sonographic assessment of diaphragmatic thickness and excursion in predicting successful extubation of preterm infants from invasive conventional mechanical ventilation. Preterm infants less than 32 weeks gestation who required invasive conventional mechanical ventilation were evaluated by diaphragmatic sonography within 1 h of their planned extubation. Infants were classified into successful or failed extubation groups based on their ability to stay off invasive mechanical ventilation for 72 h after extubation. Inspiratory and expiratory thickness plus excursion of the right and left hemidiaphragm as well as diaphragmatic thickening fraction (DTF) measures were compared between groups. We included 43 eligible infants, of whom 34 infants succeeded and 9 infants failed extubation. Infants in the successful extubation group had a significantly higher expiratory thickness of the right and left hemidiaphragm, excursion of the right and left hemidiaphragm, inspiratory thickness of the left hemidiaphragm, and DTF of the left hemidiaphragm compared with infants who failed extubation. The receiver-operating characteristic curves showed that excursion of the right and left hemidiaphragm has the highest significant accuracy in predicting successful extubation of preterm infants among all diaphragmatic parameters (AUC is 0.98 and 0.96, respectively; p value < 0.001 for both).
Conclusion : We conclude that diaphragmatic excursion is a useful indicator for successful extubation of preterm infants from mechanical ventilation.
Similar content being viewed by others

Acute dyspnea in the emergency department: a clinical review

Effect of aggressive vs conservative screening and confirmatory test on time to extubation among patients at low or intermediate risk: a randomized clinical trial
Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives.
Avoid common mistakes on your manuscript.
Introduction
The decision to extubate preterm infants from mechanical ventilators is mainly based on clinical assessment, blood gases, and ventilator settings [ 7 ]. Researchers attempted to evaluate different parameters as predictors for successful extubation of preterm infants from mechanical ventilation [ 26 , 28 ]. However, up to 30% of preterm infants who are extubated based on the clinical assessment require re-intubation indicating a poor correlation with infants’ readiness for extubation [ 3 , 28 ].
The diaphragm represents the main respiratory muscle in infancy. It contributes to generation of an estimated three-fourths of the infant's tidal volume during resting inspiration in the supine position [ 23 ]. Continuous positive airway pressure (CPAP) affects the crura of diaphragm by shortening the muscle and decreasing excursion through maintaining end expiratory lung volume [ 21 ]. Moreover, prolonged mechanical ventilation triggers myofibrillar contractile dysfunction and myofilament protein loss of the diaphragmatic muscles which later results in loss of diaphragmatic force-generating capacity, poor activity, and unloading of the diaphragm [ 10 ]. This phenomenon of ventilator-induced diaphragmatic dysfunction (VIDD) has raised the attention of investigators to the correlation between duration of mechanical ventilation and failure to extubate preterm infants from mechanical ventilation [ 27 ]. However, the diagnosis of this diaphragmatic dysfunction can be hindered by the lack of appropriate quantitative assessments of neonatal diaphragm function [ 1 ].
Accurate assessment of diaphragm function in the neonate could aid to the diagnosis of respiratory distress, evaluation of therapeutic interventions, and identification of infants ready to wean from mechanical ventilation [ 20 ]. Monitoring the electrical activity of the diaphragm in infants and children has shown that higher diaphragmatic activity in relation to tidal volume indicates a better preserved diaphragmatic function and a better chance of passing the extubation readiness test [ 29 ]. However, the tools needed for monitoring the electrical activity of the diaphragm are invasive, expensive, and require trained personnel for proper interpretation. Sonographic evaluation of the diaphragm is ubiquitous in medical facilities, requires no radiation, can be used at the infant’s bedside, and useful in assessing diaphragmatic mobility and excursion [ 13 ].
We hypothesized that assessment of diaphragmatic dimensions and excursion, before planned extubation, may be helpful in predicting successful extubation of preterm infants from mechanical ventilation. We aimed to study sonographic assessment of the diaphragmatic dimensions and excursion for mechanically ventilated preterm infants as a predictor for success of extubation.
The present study was placed at the Neonatal Intensive Care Unit (NICU) of Mansoura University Children’s Hospital, Mansoura, Egypt, between January 2017 and November 2019. The study was approved by the Institutional Review Board, Mansoura Faculty of Medicine, and a fully informed written consent was obtained from the parent or infant's guardian before enrolment in the study.
Study designs
This was a prospective, observational, cohort study assessing diaphragmatic thickness and excursion for preterm infants prior to planned extubation from invasive conventional mechanical ventilation.
Participants
Preterm infants less than 32 weeks gestation who were supported by invasive conventional mechanical ventilation for a diagnosis of respiratory distress syndrome, as evident by clinical and radiological findings, and planned for extubation were eligible for this study. Preterm infants with chromosomal aberrations, hepatosplenomegaly, pleural effusion, congenital heart or lung disorders, or congenital anomalies related to diaphragm as diaphragmatic hernia and diaphragmatic paralysis were excluded from the study.
Intervention
Eligible preterm infants had sonographic assessment of diaphragmatic thickness and excursion within 1 h of planned extubation from invasive conventional mechanical ventilation to non-invasive respiratory support. Preterm infants were extubated from mechanical ventilation if they fulfilled the following criteria: spontaneous respiratory effort, presence of cough or gag induced by suctioning, acceptable arterial blood gases (pH more than 7.25, PaCO 2 less than 60 mmHg, and base deficit less than 8 mEq/L) on a mean airway pressure less than 8 cm H 2 O and respiratory rate of less than 30/min, saturation more than 90% on fraction of inspired oxygen (FiO 2 ) less than 30% in the preceding 24 h, and the decision of extubation was taken by the attending physician who was blinded to the results of sonographic measurements.
Sonographic diaphragmatic parameters were measured while infants were on spontaneous pressure support ventilation mode with a support pressure of 4 cm H 2 O over an end expiratory pressure of 4 cm H 2 O for 1 h prior to the sonographic assessment as an accommodation. The total duration on pressure support ventilation mode and sonographic diaphragmatic assessment was 2 h at most to avoid infant exhaustion. Sonographic evaluation was performed prior to the time of next feed, while infant’s stomach is empty, to avert any interference of a full stomach on diaphragmatic mobility and measurements. All infants were extubated to nasal CPAP using the Infant Nasal CPAP Assembly system (Fisher & Paykel Healthcare, Auckland, New Zealand) at a pressure of 5 cmH 2 O and FiO 2 between 21% and 30% to keep infant's saturation between 90% and 95%.
Sonographic diaphragmatic assessment technique
Ultrasonographic examinations were performed by one operator, who had ten years of experience in diaphragm sonography, using a portable Doppler ultrasonography (Micro-Maxx; SonoSite, Bothell, WA, USA) with a micro-convex transducer array (10 to 5 MHz). Diaphragmatic sonography was performed while the infant is in supine position after ensuring quiet regular breathing. For visualization of the right hemidiaphragm, the convex probe was placed over the right subcostal and lower intercostal spaces between the anterior axillary and the midclavicular lines with the probe directed cranially, dorsally, and medially so the radial beam came to be perpendicular to the posterior third of the right hemidiaphragm. For visualization of the left hemidiaphragm, the same technique, position, and direction of the probe as the right hemidiaphragm were performed apart from the probe was placed between the left anterior axillary and midaxillary lines.
At first, the two-dimensional mode was screened to detect the appropriate exploration image for each hemidiaphragm in which the diaphragm appeared as a hypoechoic line that was placed between two echogenic lines, the upper one was the reflection of the parietal pleura and the lower was for the peritoneum. After that, the M-mode ultrasonography was screened and the image was frozen after ensuring regular up and down movement of the diaphragmatic line that reflects regular breathing. The thickness of the diaphragmatic line during inspiration (upward slope) and expiration (downward slope) represents the diaphragmatic inspiratory and expiratory thickness, respectively. The perpendicular distance between the most caudal point of this line during inspiration and the most caudal point during expiration represents the diaphragmatic excursion. Diaphragmatic thickness and kinetics measures can be affected by the irregular breathing pattern, high respiratory rate, breath to breath variability, and small diaphragmatic dimensions by using M-mode technique in preterm infants. To overcome this technical limitation, the investigator observed for regular up and down movements of the diaphragmatic line in M-mode and cine for 1 min to ensure an epoch of quiet regular breathing, and then diaphragmatic measurements were obtained [ 2 ]. The technique and measurements were repeated up to 3 respiratory cycles for each hemidiaphragm, and the average one was recorded [ 5 ]. Avoidance of diaphragmatic measurements during infant’s crying or sighing movement was taken in consideration.
The diaphragmatic thickening fraction (DTF) was calculated and recorded using the following formula: DTF = [(inspiratory thickness − expiratory thickness) / expiratory thickness] × 100 [ 8 ].
The intra-observer reproducibility was evaluated by repeated measurements of sonographic diaphragmatic parameters by the same investigator with 30 min in between measurements. Ten clinically stable preterm infants, age and sex cross-matched with the studied group, were randomly selected for this purpose.
Study end point
The primary study end point was successful extubation from invasive mechanical ventilation defined as being off mechanical ventilation with transmission to oxygen therapy or non-invasive respiratory support, for at least 72 h post-extubation [ 12 ]. The indications for re-intubation were specified as follows: more than six episodes of apnea requiring stimulation within 6 h, or more than one significant episode of apnea requiring bag and mask ventilation, respiratory acidosis (PaCO 2 > 65 mmHg and pH < 7.25) or FiO 2 > 60% to maintain saturation in the target range (90–95%) [ 12 ].
Sample size calculation
Sample size calculation was based on the area under the receiver-operating characteristic curve that was 0.79 for predicting successful extubation of mechanical ventilation retrieved from previous research [ 4 ]. Using MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium), sample size calculation using area under ROC curves with null hypothesis = 0.5, α-error = 0.05, power of the study = 80% ratio of positive to negative cases which will be considered as 3.3. The total calculated sample size will be 43 cases.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (version 21; IBM Corporation, Armonk, NY, USA). Student’s t test was used to compare continuous parametric variables. Mann–Whitney U test was used for continuous non-parametric variables. Chi-square test or Fisher’s exact test was used for categorical variables when appropriate. Shapiro–Wilk test was done to examine the distribution of data. Pearson's correlation coefficient test was used to correlate between duration of invasive mechanical ventilation and different diaphragmatic measures. The accuracy of different diaphragmatic measurements for predicting successful extubation from invasive mechanical ventilation was evaluated using receiver-operating characteristic (ROC) curves. A p value of < 0.05 is considered to be statistically significant. Data are expressed as mean ± standard deviation, median (inter-quartile range), or number (percentage) unless otherwise stated. Reproducibility of the diaphragmatic measurements was assessed by Bland–Altman analysis and Pearson’s correlation coefficient.
Of the 164 preterm infants who were at less than 32 weeks gestation admitted to our NICU during the study period, 62 infants required invasive conventional mechanical ventilation, 43 were included in the study, and 19 were excluded due to various causes (Fig. 1 ). A total of 34 infants succeeded and 9 infants failed extubation. Infants in the failed extubation group had significantly higher postnatal age at extubation, longer duration of invasive mechanical ventilation, higher pre-extubation mean airway pressure, and higher pre-extubation FIO 2 compared with infants in the successful extubation group (Table 1 ). Of the 9 infants who failed extubation, 6 infants were reintubated for increased work of breathing in association with hypoxia, two infants were reintubated for increased work of breathing in association with hypercapnia, and one infant was reintubated for apnea which was preceded by increased work of breathing. The mean time for reintubation was 43.5 ± 13.5 h with a minimum of 29 and a maximum of 66 h, respectively. Measurements were highly reproducible with a high degree of agreement between diaphragmatic dimensions as assessed by Pearson’s correlation coefficient and Bland–Altman analysis. Pearson’s correlation coefficient values were above 0.9, and p values were < 0.001 for all measured diaphragmatic indices.
Diagram showing the flow of participants in the study
Infants in the successful extubation group had a significantly higher expiratory thickness of the right and left hemidiaphragm, excursion of the right and left hemidiaphragm, inspiratory thickness of the left hemidiaphragm, and DTF of the left hemidiaphragm compared with infants who failed extubation to nasal CPAP (Table 2 ) (Fig. 2 ). The duration of invasive mechanical ventilation had a significant negative correlation with inspiratory and expiratory thickness of the right and left hemidiaphragm, excursion of the right hemidiaphragm, and DTF of the right and left hemidiaphragm (Table 3 ).
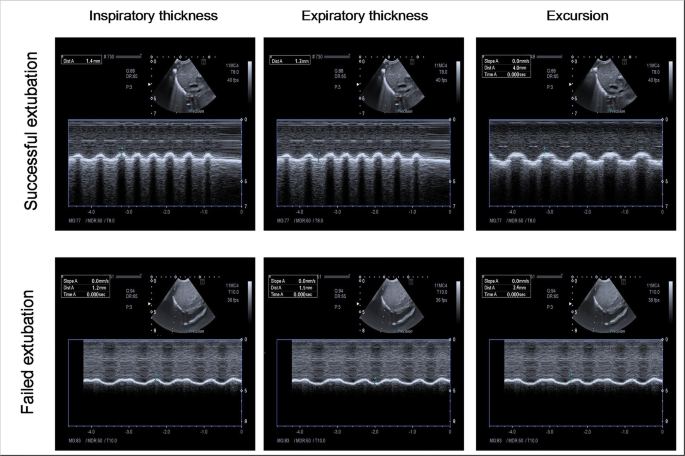
Sonographic images showing M-mode measurements of inspiratory thickness, expiratory thickness, and excursion of the right hemidiaphragm in an infant (case number 5) from the successful extubation group and an infant (case number 11) from the failed extubation group
The ROC curves showed that expiratory thickness of the right and left hemidiaphragm, excursion of the right and left hemidiaphragm, inspiratory thickness of the left hemidiaphragm, and DTF of the left hemidiaphragm had significant accuracies in predicting successful extubation of preterm infants (Fig. 3 ). Excursion of the right and left hemidiaphragm showed the highest accuracy among all diaphragmatic parameters. A right hemidiaphragmatic excursion of 2.75 mm was associated with 94% sensitivity and 89% specificity in predicting successful extubation. A left hemidiaphragmatic excursion of 2.45 mm was associated with 94% sensitivity and 89% specificity in predicting successful extubation.
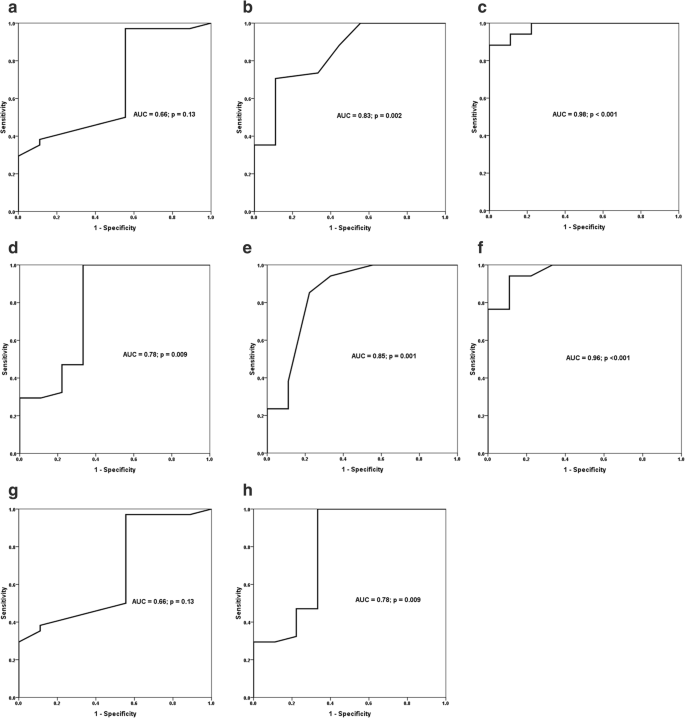
Receiver-operating characteristic curves show area under the curve (AUC) and p value of significance for inspiratory thickness of the right hemidiaphragm ( a ), expiratory thickness of the right hemidiaphragm ( b ), excursion of the right hemidiaphragm ( c ), inspiratory thickness of the left hemidiaphragm ( d ), expiratory thickness of the left hemidiaphragm ( e ), excursion of the left hemidiaphragm ( f ), and diaphragm thickening fraction (DTF) of the right hemidiaphragm ( g ) and the left hemidiaphragm ( h ) in predicting successful extubation of preterm infants from mechanical ventilation
Sonographic assessment of the lungs and diaphragm has gained the interest of neonatologists nowadays. Sonographic assessment of the lungs has shown a high sensitivity and specificity in diagnosing various respiratory disorders in neonates [ 24 ]. Ultrasound has been recently used to assess diaphragmatic thickness and excursion of diaphragmatic dome in stable spontaneously breathing infants [ 5 ]. We aimed to assess the accuracy of sonographic assessment of diaphragmatic thickness and excursion as a predictor for successful extubation of preterm infant from invasive conventional mechanical ventilation. The main finding of our study is that excursion of the right and left hemidiaphragm has the highest accuracy in predicting successful extubation of mechanically ventilated preterm infants. Diaphragmatic excursion was significantly higher in preterm infants successfully extubated from invasive conventional mechanical ventilation compared with infants who failed extubation.
Diaphragmatic activity as a predictor for successful extubation was evaluated in pediatric age group through monitoring of diaphragmatic electrical activity. Assessment of diaphragmatic electrical activity has shown that infants and children who generated higher diaphragmatic activity in relation to tidal volume had a better chance of passing the extubation readiness test as opposed to infants and children who generated lower diaphragmatic activity in relation to tidal volume [ 29 ]. Authors in this study stated that diaphragmatic activity in relation to tidal volume indicates a better preserved diaphragmatic function [ 29 ].
To the best of our knowledge, this study is the earliest to report the accuracy of assessing diaphragmatic activity by using diaphragmatic ultrasound in prediction of successful extubation in preterm infants. Over 400 participants between 1 month and 16 years, sonographic assessment of the diaphragm has shown a high accuracy in assessing diaphragmatic thickness and excursion [ 5 ]. Rehan and colleagues reported normal diaphragmatic excursion in 34 preterm infants between 26 and 37 weeks gestation to be 5.5 ± 0.2 mm at 26 to 28 weeks gestation, 4.8 ± 0.2 mm in 29 to 31 weeks gestation, 4.6 ± 0.2 mm in 32 to 34 weeks gestation, and 4.4 ± 0.3 mm in 35 to 37 weeks gestation [ 22 ]. The difference between our measurements and Rehan's study is attributed to their inclusion of clinically stable preterm infants who have no evidence of any acute illness, no culture proven sepsis, not on any oxygen supplementation, and not on CPAP or ventilator support compared with our ventilated infants. Radicioni and colleagues tested the accuracy of a model that consists of the sonographic measurements of right diaphragmatic excursions during inspiration and expiratory phases plus the oxygen saturation/FiO 2 ratio as a predictor for CPAP failure in preterm infants with respiratory distress syndrome. The authors found that integration of both measures in this model has a high accuracy, with AUC 0.95, in predicting CPAP failure [ 19 ].
In mechanically ventilated adults, sonographic assessment of diaphragmatic function showed that diaphragmatic excursion was significantly higher in the successful group compared with those who failed extubation [ 6 ]. Liu and colleagues found that diaphragmatic excursion had a sensitivity of 89.2% and a specificity of 75.0% with an AUC (ROC) of 0.849 in predicting successful extubation in mechanically ventilated adult patients. The cut-off value of diaphragmatic excursion for predicting successful extubation was determined to be 1.14 cm by ROC curve analysis [ 16 ]. Yoo et al. found that diaphragmatic excursion is more accurate than a change in the diaphragm thickness to predict extubation success in mechanically ventilated adults [ 31 ]. In a meta-analysis of 13 studies over 742 adults, Li and colleagues concluded that diaphragmatic excursion and thickness are accurate measures for predicting reintubation within 48 h of extubation despite having a large heterogeneities among the included studies [ 15 ].
In mechanically ventilated adults, McCool and colleagues [ 17 ] showed that the duration of mechanical ventilation was significantly shorter in patients diagnosed with normal diaphragmatic function as assessed by ultrasound measurement of diaphragmatic thickness and excursion. The authors stated that normal diaphragmatic function as assessed by ultrasound shows 90.9% sensitivity, 86.7% specificity, 90.9% positive predictive value, and 86.7% negative predictive value in predicting successful extubation from mechanical ventilation [ 17 ].
The proposed mechanism for diaphragmatic dysfunction in association with invasive mechanical ventilation is the loss of myofilament protein of diaphragmatic muscle which results in what is known as ventilator-induced diaphragmatic dysfunction (VIDD). A previous research revealed that only 18–24 h of invasive mechanical ventilation is sufficient to develop VIDD in both laboratory animals and humans [ 18 ]. In animal models, ventilator-induced diaphragmatic proteolysis and associated diaphragmatic atrophy occur due to increased diaphragmatic protein breakdown and decreased protein synthesis which is mediated by various proteases, such as calpain, caspase-3, autophagy, and the ubiquitin-proteasome system [ 18 ]. We have found that expiratory thickness of the right and left hemidiaphragm and inspiratory thickness of the left hemidiaphragm were significantly lower in infants who failed compared with infants who succeeded extubation from mechanical ventilation. Our results support previous results of ventilator-induced diaphragmatic atrophy which were retrieved by animal studies and human studies [ 11 , 14 ]. This is further supported by our finding of negative correlation between the duration of mechanical ventilation with inspiratory and expiratory thickness of the right and left hemidiaphragm, excursion of the right hemidiaphragm, and DTF of the right and left hemidiaphragm. The possible cause of the absence of significant difference in the inspiratory thickness and thickening fraction of the right hemidiaphragm between successfully and failed extubated preterm infants can be attributed to the supporting effect of the liver to the right hemidiaphragm during inspiration which can mask minimal effects on the muscle mass of the right hemidiaphragm. Another possibility for this non-significant difference may be related to our use of M-mode technique during measurement. Compared with B-mode, M-mode may not obtain reliable measurements of the diaphragmatic thickness, due to the subtlety of the imaging line. However, we compensated for this by obtaining our M-mode measures over the most moving point of the hemidiaphragm on B-mode. Moreover, M-mode technique has been successfully used in previous studies to measure diaphragmatic thickness in excursion in adults and pediatric age groups [ 9 , 30 ].
A potential technical limitation to our study is the use of a micro-convex transducer rather than a high-frequency micro-linear transducer for imaging. The latter has better ability for visualization of the thin muscles and superficial structures, like hemidiaphragm. However, the micro-convex transducer gives a better in-between ribs view and wider angle of image “pie-shaped image” of the whole hemidiaphragm which allows for better identification of the most moving part of the hemidiaphragm in B-mode. We compensated for this technical limitation by taking our M-mode measurements at the most moving part of hemidiaphragm in the B-mode view.
We acknowledge that the study is limited by the relatively small sample size, which is attributed to our adoption of the early administration of non-invasive respiratory support techniques to our preterm infants to minimize ventilator-induced lung injury which decreased the percentage of preterm infants who required invasive mechanical ventilation during the study period. The study is also limited by the lack of physiopathologic proof of respiratory etiology as a cause for extubation failure and the need for reintubation. It is of note that assessment of diaphragmatic function in infants who failed extubation due to non-respiratory causes, such as central apnea or poor respiratory drive, is less valuable. Moreover, our practice of extubation to a nasal CPAP of 5 cm H 2 O represents another limitation given the new guidelines of extubating preterm infants to CPAP pressure of 7–9 cm H 2 O [ 25 ]. Our low level of CPAP support may have resulted to an increased incidence of preterm infants who required reintubation. Future studies should consider evaluation of diaphragmatic thickness and excursion in relation of different modes and parameters of respiratory support to find out the appropriate approach for respiratory support in preterm infants that maintain adequate diaphragmatic stimulation and prevent VIDD.
In conclusion, sonographic assessment of diaphragmatic thickness and excursion represents a promising sensitive and specific easily applicable tool to predict successful extubation of preterm infants from invasive conventional mechanical ventilation.
Abbreviations
Area under the curve
Continuous positive airway pressure
Diaphragmatic thickening fraction
Neonatal intensive care unit
Ventilator induced diaphragmatic dysfunction
Fraction of inspired oxygen
Bhat RY, Greenough A, Rafferty GF, Patel S, Chandler C (2004) Assessment of diaphragm function in lumbocostovertebral syndrome. Eur J Pediatr 163:694–695
PubMed Google Scholar
Buonsenso D, Musolino A (2018) Point of care diaphragm ultrasound in infants with bronchiolitis. Pediatr Pulmonol 53:1597
Article Google Scholar
Dimitriou G, Greenough A, Endo A, Cherian S, Rafferty GF (2002) Prediction of extubation failure in preterm infants. Arch Dis Child Fetal Neonatal Ed 86:F32–F35
Article CAS Google Scholar
DiNino E, Gartman EJ, Sethi JM, McCool FD (2013) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69:423–427
El-Halaby H, Abdel-Hady H, Alsawah G, Abdelrahman A, El-Tahan H (2016) Sonographic evaluation of diaphragmatic excursion and thickness in healthy infants and children. J Ultrasound Med 35:167–175
Farghaly S, Hasan AA (2017) Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care 30:37–43
Fox WW, Schwartz JG, Shaffer TH (1981) Successful extubation of neonates: clinical and physiological factors. Crit Care Med 9:823–826
Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, Brochard LJ, Bolz SS, Rubenfeld GD, Kavanagh BP, Ferguson ND (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 41:734
Huang D, Ma H, Zhong W, Wang X, Wu Y, Qin T, Wang S, Tan N (2017) Using M-mode ultrasonography to assess diaphragm dysfunction and predict the success of mechanical ventilation weaning in elderly patients. J Thorac Dis 9:3177–3186
Hussain SN, Cornachione AS, Guichon C, Al Khunaizi A, Leite Fde S, Petrof BJ, Mofarrahi M, Moroz N, de Varennes B, Goldberg P, Rassier DE (2016) Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax 71:436–445
Jaber S, Jung B, Matecki S, Petrof BJ (2011) Clinical review: ventilator-induced diaphragmatic dysfunction—human studies confirm animal model findings! Crit Care 15:206
Kamlin CO, Davis PG, Morley CJ (2006) Predicting successful extubation of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 91:F180–F183
Kantarci F, Mihmanli I, Demirel MK, Harmanci K, Akman C, Aydogan F, Mihmanli A, Uysal O (2004) Normal diaphragmatic motion and the effects of body composition: determination with M-mode sonography. J Ultrasound Med 23:255–260
Lee EP, Hsia SH, Hsiao HF, Chen MC, Lin JJ, Chan OW, Lin CY, Yang MC, Liao SL, Lai SH (2017) Evaluation of diaphragmatic function in mechanically ventilated children: an ultrasound study. PLoS One 12:e0183560
Li C, Li X, Han H, Cui H, Wang G, Wang Z (2018) Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore) 97:e10968
Liu LX, Su D, Hu ZJ (2017) The value of the excursion of diaphragm tested by ultrosonography to predict weaning from mechanical ventilation in ICU patients. Zhonghua Nei Ke Za Zhi 56:495–499
CAS PubMed Google Scholar
McCool FD, Oyieng'o DO, Koo P (2020) The utility of diaphragm ultrasound in reducing time to extubation. Lung 198:499–505
Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ (2013) Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 305:R464–R477
Radicioni M, Leonardi A, Lanciotti L, Rinaldi VE, Bini V, Camerini PG (2020) How to improve CPAP failure prediction in preterm infants with RDS: a pilot study. Eur J Pediatr: [published online ahead of print, 2020 Jun 19]. Eur J Pediatr. https://doi.org/10.1007/s00431-020-03700-w
Rafferty GF, Greenough A, Dimitriou G, Kavadia V, Laubscher B, Polkey MI, Harris ML, Moxham J (2000) Assessment of neonatal diaphragm function using magnetic stimulation of the phrenic nerves. Am J Respir Crit Care Med 162:2337–2340
Rehan VK, Laiprasert J, Nakashima JM, Wallach M, McCool FD (2001) Effects of continuous positive airway pressure on diaphragm dimensions in preterm infants. J Perinatol 21:521–524
Rehan VK, Laiprasert J, Wallach M, Rubin LP, McCool FD (2001) Diaphragm dimensions of the healthy preterm infant. Pediatrics 108:E91
Shaffer TH, Wolfson MR, Bhutani VK (1981) Respiratory muscle function, assessment, and training. Phys Ther 61:1711–1723
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D (2020) International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 24:65
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL (2019) European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology 115:432–450
Tapia-Rombo CA, Galindo-Alvarado AM, Saucedo-Zavala VJ, Cuevas-Uriostegui ML (2007) Predictive factors of failure extubation among preterm infants. Gac Med Mex 143:101–108
Vassilakopoulos T, Petrof BJ (2004) Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 169:336–341
von Merkel J, Gebauer C, Blaser A, Pulzer F, Thome U, Knupfer M (2012) Prediction of extubation failure in ELBW preterm infants. Klin Padiatr 224:324–330
Wolf GK, Walsh BK, Green ML, Arnold JH (2011) Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med 12:e220–e224
Xue Y, Zhang Z, Sheng CQ, Li YM, Jia FY (2019) The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm Med 19:270
Yoo JW, Lee SJ, Lee JD, Kim HC (2018) Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success. Korean J Intern Med 33:331–339
Download references
Author information
Authors and affiliations.
Neonatal Intensive Care Unit, Mansoura University Children’s Hospital, Mansoura, Egypt
Eslam Bahgat, Nehad Nasef & Hesham Abdel-Hady
Department of Pediatrics, Faculty of Medicine, University of Mansoura, Mansoura, Egypt
Hanan El-Halaby, Nehad Nasef & Hesham Abdel-Hady
Department of Diagnostic Radiology, Mansoura University Children’s Hospital, Mansoura, Egypt
Ashraf Abdelrahman
Department of Pediatrics, Mansoura University Children’s Hospital, Gomhoria Street, Mansoura, 35516, Egypt
Nehad Nasef
You can also search for this author in PubMed Google Scholar
Contributions
Eslam Bahgat and Hanan El-Halaby participated in the design of the study, data collection, and writing the manuscript. Ashraf Abdelrahman participated in sonographic assessment of the diaphragm and manuscript writing. Nehad Nasef and Hesham Abdel-Hady participated in formulating the hypothesis, design of the study, data collection, data interpretation, statistical analysis, and writing of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Correspondence to Nehad Nasef .
Ethics declarations
Conflict of interests.
The authors declare that they have no conflict of interest.
Ethical approval
This article has been approved by the Institutional Review Board (IRB), Mansoura Faculty of Medicine, Mansoura, Egypt.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Bahgat, E., El-Halaby, H., Abdelrahman, A. et al. Sonographic evaluation of diaphragmatic thickness and excursion as a predictor for successful extubation in mechanically ventilated preterm infants. Eur J Pediatr 180 , 899–908 (2021). https://doi.org/10.1007/s00431-020-03805-2
Download citation
Received : 05 July 2020
Revised : 22 August 2020
Accepted : 08 September 2020
Published : 28 September 2020
Issue Date : March 2021
DOI : https://doi.org/10.1007/s00431-020-03805-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Preterm infant
- Mechanical ventilation
- Extubation predictor
- Ultrasound waves
- Find a journal
- Publish with us
- Track your research
Diaphragmatic excursion by ultrasound: reference values for the normal population; a cross-sectional study in Egypt
Affiliations.
- 1 Chest Diseases Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
- 2 Chest Diseases Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt.
- 3 College of Medical Rehabilitation Sciences, Taibah University, Medina, Saudi Arabia.
- 4 Mahatma Gandhi University, Meghalaya, India.
- PMID: 35756096
- PMCID: PMC9220962
- DOI: 10.4081/mrm.2022.842
Background: Measurement of diaphragmatic motion by ultrasound is being utilized in different aspects of clinical practice. Defining reference values of the diaphragmatic excursion is important to identify those with diaphragmatic motion abnormalities. This study aimed to define the normal range of diaphragmatic motion (reference values) by Mmode ultrasound for the normal population.
Methods: Healthy volunteers were included in this study. Those with comorbidities, skeletal deformity, acute or chronic respiratory illness were excluded. Diaphragmatic ultrasound in the supine position was performed using a lowfrequency probe. The B-mode was applied for diaphragmatic identification, and the M-mode was employed for the recording of the amplitude of diaphragm contraction during quiet breathing, deep breathing and sniffing.
Results: The study included 757 healthy subjects [478 men (63.14%) and 279 women (36.86%)] with normal spirometry and negative history of previous or current respiratory illness. Their mean age and BMI were 45.17 ±14.84 years and 29.36±19.68 (kg/m 2 ). The mean right hemidiaphragmatic excursion was 2.32±0.54, 5.54±1.26 and 2.90±0.63 for quiet breathing, deep breathing and sniffing, respectively, while the left hemidiaphragmatic excursion was 2.35±0.54, 5.30±1.21 and 2.97±0.56 cm for quiet breathing, deep breathing and sniffing, respectively. There was a statistically significant difference between right and left diaphragmatic excursion among all studied subjects. The ratio of right to left diaphragmatic excursion during quiet breathing was (1.009±0.19); maximum 181% and minimum 28%. Only 19 cases showed a right to left ratio of less than 50% (5 men and 14 women). The diaphragmatic excursion was higher in males than females. There was a significant difference in diaphragmatic excursion among age groups. Age, sex and BMI significantly affected the diaphragmatic motion.
Conclusions: Diaphragmatic excursion values presented in this study can be used as reference values to detect diaphragmatic dysfunction in clinical practice. Diaphragmatic motion is affected by several factors including age, sex and body mass index.
Keywords: M-mode ultrasound; diaphragmatic excursion; diaphragmatic motion; diaphragmatic ultrasound; normal values; reference values.
©Copyright: the Author(s).
- Research article
- Open access
- Published: 27 January 2023
Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease
- Taehwa Kim 1 , 2 na1 ,
- Sungchul Huh 3 na1 ,
- Jae Heun Chung 1 , 2 ,
- Yun Seong Kim 1 , 2 ,
- Ra Yu Yun 3 , 4 ,
- Onyu Park 5 &
- Seung Eun Lee ORCID: orcid.org/0000-0002-4266-7722 1 , 2
BMC Pulmonary Medicine volume 23 , Article number: 33 ( 2023 ) Cite this article
2139 Accesses
1 Citations
1 Altmetric
Metrics details
The limitation of activity due to dyspnea in chronic obstructive pulmonary disease (COPD) patients is affected by diaphragmatic dysfunction and reduced lung function. This study aimed to analyze the association between diaphragm function variables and forced expiratory volume in the first second (FEV1) and to estimate the clinical significance of diaphragm function in the correlation between COPD severity and lung function.
This prospective, single-center, cross-sectional observational study enrolled 60 COPD patients in a respiratory outpatient clinic. Data for baseline characteristics and the dyspnea scale were collected. Participants underwent a pulmonary function test (PFT), a 6-minute walk test (6MWT), and diaphragm function by ultrasonography.
The right excursion at forced breathing showed the most significant correlation with FEV1 ( r = 0.370, p = 0.004). The cutoff value was 6.7 cm of the right diaphragmatic excursion at forced breathing to identify the FEV1 above 50% group. In the group with a right diaphragmatic excursion at forced breathing < 6.7 cm, modified Medical Research Council (mMRC), St. George's Respiratory Questionnaire and the total distance of 6MWT showed no difference between groups with FEV1 under and above 50% ( p > 0.05). In the group with ≥ 6.7 cm, mMRC and the total distance of 6MWT showed a significant difference between FEV1 under and above 50% ( p = 0.014, 456.7 ± 69.7 m vs. 513.9 ± 60.3 m, p = 0.018, respectively).
The right diaphragmatic forced excursion was closely related to FEV1, and analysis according to the right diaphragmatic forced excursion-based cut-off value showed a significant difference between both groups. When the diaphragm function was maintained, there was a lot of difference in the 6MWT’s factors according to the FEV1 value. Our data suggest that diaphragmatic function should be performed when interpreting PFT.
Peer Review reports
Introduction
The most common complaint in respiratory diseases regardless of the disease type is dyspnea [ 1 ]. COPD is characterized by worsening dyspnea during movement [ 2 ]. COPD restricts various activities of daily living due to shortness of breath, leading to poor quality of life and increased mortality and morbidity [ 3 ]. There are many causes of dyspnea; however, for patients with stable COPD, a major contributor is the weakening of the respiratory muscles, excluding conditions such as acute infectious diseases [ 4 ].
The diaphragm is the main respiratory muscle, particularly the inspiratory muscles. The weakness of the diaphragm in COPD has been extensively studied. Some studies have reported a significant reduction in diaphragmatic excursion in patients with COPD [ 5 , 6 , – 7 ]. Lung hyperinflation-associated shortening of the diaphragm has traditionally been considered a major cause of diaphragmatic weakness [ 8 ]. Also, there were previous studies about diaphragmatic thickness. Diaphragmatic thickness was a factor related to weaning and prognosis in patients under mechanical ventilation [ 9 , 10 ]. Recently, several studies have reported the clinical value of diaphragm ultrasonography according to COPD severity, and even compared to traditional methods, the diagnostic value of ultrasonography has proven to be reliable and useful [ 11 ]. Ultrasonography is also commonly used in medical facilities because it can be carried out anywhere, has no associated radiation risk, and can be used to adequately visualize the structure of the diaphragm [ 12 ].
Furthermore, 6MWT is an important tool for assessing exercise capacity and functional status in patients with COPD. Diaphragmatic weakness can impair physical performance, especially the 6MWT [ 13 , 14 ]. A previous study reported that pulmonary function was significantly correlated with the 6MWT in patients with severe and very severe COPD [ 15 ]. The relationship between 6MWT and PFT is a matter of connecting and understanding the respiratory muscles. PFT is used to measure the volume and flow rate of the lungs, and 6MWT is an important test for evaluating the exercise capacity and functional status of patients.
When we summarize the above, PFT correlates with 6MWT in COPD patients [ 15 ]. 6MWT can evaluate physical performance of COPD patients. Physical performance can also reflect diaphragmatic weakness [ 13 , 14 ]. Therefore, PFT correlates with 6MWT, 6MWT reflects physical performance, and physical performance was associated with diaphragmatic weakness. This relationship of PFT and diaphragmatic weakness can be expressed as follows for the patient. If the pulmonary function expressed by PFT is good, or if case which the power and strength of the respiratory muscles are good when PFT remains the same, breathing is more stable. Therefore, understanding the physiological principles of the respiratory muscle performance that establish the relationship these and compensate for this is important for managing the patient’s condition. Through this study, a review of the correlation between the PFT reflecting the 6MWT and diaphragm ultrasound features of respiratory muscle may be helpful to understand the physiological principles of patients with COPD.
Thus, this study aimed to analyze diaphragm movement characteristics using ultrasonography in patients with COPD and clarify its association with pulmonary function.
Study design and methods
Study design and participants.
This single-center, prospective, cross-sectional observational study recruited participants from a tertiary hospital outpatient respiratory clinic between April 2020 and April 2021. The inclusion criteria were: 1) patients 18 years old or older diagnosed with COPD by a pulmonologist; COPD diagnostic criterion was a post-bronchodilator FEV1/forced vital capacity (FVC) ratio < 0.70 based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2) patients who could maintain the required posture for diaphragm function measurement by ultrasonography and stable breathing during the examination such as 6MWT. Patients unable to cooperate with the examination and unstable patients requiring immediate medical intervention were excluded. Patients with interstitial lung disease featured on chest computed tomography (CT) that could affect diaphragm movement were also excluded.
Sixty-nine patients were enrolled, six of whom with combined interstitial lung disease on CT were excluded. Two patients were lost to follow-up, and one died before all examinations were completed. Finally, 60 patients completed all examinations for the study protocol and were included in the analysis.
All patients provided informed consent before participating in the study. Each patient’s clinical information was collected from four domains: pulmonary function, exercise capacity, body composition, and diaphragm function. Pulmonary function was evaluated through spirometry, MIP, and maximal expiratory pressure (MEP). Exercise capacity and body composition were assessed using the 6MWT and bioelectrical impedance analysis (BIA). Diaphragm dysfunction is defined as loss of muscle contractility [ 16 ]. To evaluated diaphragm dysfunction, we was assessed using ultrasonography in both the M-mode and B-mode for excursion and thickness, respectively.
Assessments
For patients who had performed a PFT within 1 month of participating in the study, the previous results were used and no retesting was performed. Patients who had no available PFT results within 1 month of participating in this study were reevaluated after enrollment. The Carefusion Vmax 20 (VIASYS Healthcare Inc. Sensormedics; Yorba Linda, CA, USA) was used for PFTs and FEV1, FVC, diffusing capacity of the lungs for CO, and total lung capacity were measured using the body plethysmography test. Regarding spirometry, the patients sat in a small booth and breathed into a mouthpiece. One technical expert from the Department of Respiratory Medicine conducted all the tests to maintain the consistency of the results.
MIP (PONY FX, COSMED Inc.; Rome, Italy) and MEP (PONY FX, COSMED Inc.; Rome, Italy) were measured in the sitting position using a portable mouth pressure meter. Three consecutive MIP and MEP measurements were taken, and the best result was recorded. The PFT was measured in a sitting position. A flanged mouthpiece was applied to the short and rigid tube of the measuring instrument and air leakage was checked around the mouthpiece before testing. The test was performed by an experienced examiner who has conducted the test for more than 8 years. MIP was measured by exhaling as deep as possible and inhaling as hard as possible for at least 1.5 s. MEP was measured by inhaling as deep as possible and exhaling as hard as possible for at least 1.5 s. Both measurements were made three times, and patients recovered to normal breathing patterns with at least a minute of break between measurements. The highest of the three measurements was recorded [ 17 ].
The 6MWT was performed according to the American Thoracic Society standards under the direction of a well-trained respiratory therapist at a 30 m indoor walking course [ 18 ]. Patients were encouraged by the instructor every minute and were allowed to rest or quit the test at any point. We measured the total distance and peripheral saturation with the portable oxygen meter. The patients’ body compositions were estimated indirectly using the BIA from a supine position (InBody S10, InBody, Co. Ltd., Seoul, Korea).
Diaphragm function was assessed using ultrasonography (LOGIQ E9, GE Healthcare; Chicago, IL, USA) obtained from both supine and sitting positions. It is generally accepted that there are positional differences in diaphragm contractility. The effects of gravitational loading on the diaphragm length-tension and body position-mediated changes in intra-abdominal pressure may explain the differences found. Not only that there is also a difference in the excursion between right and left. The excursion of the right diaphragm shows a lower value than that of the left diaphragm because the liver in the abdominal cavity restricts the movement of the right diaphragm. We also measured the diaphragm function in two positions based on this information. The supine position involved lying on the back or with the face upward while the sitting position was semi-seated (45–60 degrees). Both M-mode and B-mode imaging were used to evaluate diaphragmatic excursion and thickness, respectively. The mid-clavicular line and the liver were used as anatomical landmarks on the right side and the spleen on the left side to visualize the diaphragm in the M-mode. B-mode ultrasonography was used to measure the diaphragmatic thickness at the bilateral zone of apposition [ 19 ]. The diaphragm thickness was measured during quiet spontaneous breathing without peak inspiratory or expiratory maneuvers. The diaphragmatic thickness fraction was calculated as the difference between thickness at the end of inspiration and thickness at the end of expiration divided by thickness at the end of expiration x 100. The diaphragmatic excursion was measured as follows. The highest position of the diaphragm movement taken by the M-mode was considered to be the end-expiratory phase, whereas the lowest position was considered as the end-inspiratory phase.
The dyspnea scale used St. George's Respiratory Questionnaire (SGRQ) and the modified Medical Research Council scale (mMRC scale). The SGRQ is a self-administered questionnaire with 76 items [ 20 ]. This can identify the patient’s symptoms and the activities of daily life. mMRC scale is most commonly used in the assessment of dyspnea in chronic respiratory diseases and is a very useful and unrecognized dyspnea scale [ 21 ].
Statistical analysis
The data were analyzed using IBM SPSS (version 27.0; Chicago, IL, USA). The level of significance was set at p < 0.05. Descriptive statistics, including numbers, percentages, means, and standard deviations, were used to summarize each variable (demographics, PFTs, 6MWT, and diaphragmatic ultrasound results). The results were analyzed by independent t-test, cross-analysis, and frequency analysis. The correlation between the variables was analyzed by Pearson’s Correlation Coefficient, which confirmed the linear relationship between two variables using a scatterplot. The cut-off value was calculated using the receiver operating characteristic (ROC) curve analysis. The reference plane was 0.5 or more in the ROC curve, and the p -value < 0.05; hence, this result was adopted. Consequently, the cut-off value was confirmed when sensitivity and specificity were plotted in a line chart, which is the point where the two graphs meet.
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed throughout this study. The study procedures were reviewed and approved by our Pusan National University Yangsan Hospital Institutional Review Board [IRB No. 05–2020-217].
FEV1 and diaphragm function
We assessed whether diaphragm function was associated with FEV1 (Fig. 1 ). In the total group analysis, both diaphragmatic excursion and thickness were associated with FEV1. However, the diaphragmatic excursion was more associated with FEV1 than thickness. Diaphragmatic excursion during forced breathing and in the supine position had a greater association with FEV1 than breathing at rest and in the sitting position. Additionally, when comparing the right and left under the same conditions, the right was more significant during forced breathing and in the supine position ( r = 0.370, p = 0.004,). Moreover, diaphragmatic thickness at right end-expiration was associated with FEV1. In summary, right ( r = 0.370, p = 0.004) and left ( r = 0.257, p = 0.048) diaphragmatic excursion during forced breathing in the supine position and diaphragmatic thickness at right end-expiration ( r = 0.310, p = 0.016) were significantly associated with FEV1.
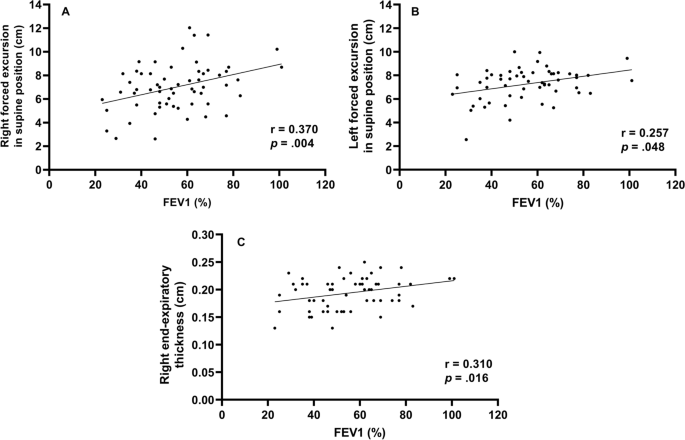
Correlation between forced expiratory volume in 1 s and diaphragm function Right forced excursion, and left forced excursion in the supine position and right end-expiratory thickness were correlated to forced expiratory volume in 1 s
Diaphragmatic function and BMI (body mass index)
To evaluate the function of the diaphragm muscle [ 22 ], the diaphragmatic excursion was measured at rest and during forced expiration (Supplement Table 1 ). In 60 patients, diaphragmatic excursion at rest in the supine position was 3.5 cm ± 1.2 on the right side and 3.5 cm ± 1.2 on the left side. During forced breathing, diaphragmatic excursion in the supine position was 6.9 cm ± 2.0 on the right side and 7.6 cm ± 1.6 on the left side. The total percent body fat was 24.2% ± 6.9. Segmental lean mass analysis was performed by direct segmental multi-frequency BIA. The lean mass was 90.5% ± 9.7 on the right arm, 88.1% ± 9.2 on the left arm, 94.5% ± 5.8 on the trunk, 95.7% ± 131.3 on the right leg, and 9.51% ± 8.8 on the left leg.
Cutoff value-associated characteristics
The ROC curve analysis of the diaphragm function variables was performed to identify the cutoff value for differentiating between FEV1 ≥ 50% and < groups. The cutoff value was ≤ 6.7 cm on the right diaphragmatic excursion at forced breathing with an area under the curve of 0.5 or more and p -value was 0.043. Right diaphragmatic excursion during forced breathing was less than the cut-off value of 6.7 cm for 26 patients and ≥ 6.7 cm for 43 patients (Table 1 ). There were no differences in age, sex, or smoking history between the two groups. The dyspnea scales such as mMRC, SGRQ, and GOLD were not significantly different between both groups. There were no differences in body mass index, percent body fat, or lean mass of the right or left legs between the groups. However, among the pulmonary function indicators, there were significant differences between the two groups. Specifically, FEV1, FVC, and MIP were significantly different (< 6.7 cm group vs. ≥ 6.7 cm group, FEV1: 49.2% ± 16.2 vs. 59.5% ± 17.2, p = 0.021; FVC: 76.2% ± 19.1 vs. 86.0% ± 15.5, p = 0.032; MIP: 67.4 cm H 2 O ± 25.1 vs. 86.5 cm H 2 O ± 28.7, p = 0.010). Concerning the 6MWT, there was a significant difference in SpO2 before 6MWT and the number of interruptions (SpO2 before 6MWT: 94.1% ± 2.7 vs. 95.3% ± 1.6, p = 0.038; number of interruptions: 4 [15.4%] vs. 0 [0%], p = 0.018). The left diaphragmatic excursion during forced breathing was also different between the two groups (6.8 cm ± 1.5 vs. 7.6 cm ± 1.3, p = 0.022) as well as the diaphragmatic thickness during right end-inspiration (0.3 cm ± 0.1 vs. 0.4 cm ± 0.1, p = 0.006). In addition, the ROC ≥ 6.7 cm group left diaphragmatic excursion was also measured with a value greater than that of the ROC < 6.7 cm group.
Subgroup characteristics according to FEV1
To identify the clinical significance of diaphragm function with the relationship between lung function, and COPD severity, the two groups classified as a right diaphragmatic excursion at 6.7 cm of forced breathing were further divided into groups based on FEV1 (< 50% or ≥ 50%) (Table 2 ). There were significant differences in age (65.0 ± 7.8 years vs. 72.7 ± 6.2 years, p = 0.011), the GOLD score ( p < 0.001), FEV1/FVC (40.1% ± 14.7 vs. 55.%4 ± 11.4, p = 0.007), peak expiratory flow rate (183.3 L/min ± 80.4 vs. 275.8 L/min ± 113.8, p = 0.027), SpO2 after the 6MWT (85.9% ± 6.5 vs. 91.5% ± 2.2, p = 0.011), and left diaphragmatic excursion during forced breathing (6.2 cm ± 1.6 vs. 7.4 cm ± 1.0, p = 0.038).
When the group with the right diaphragmatic excursion ≥ 6.7 cm was further divided into subgroups according to FEV1 (< 50% or ≥ 50%) and analyzed, mMRC, GOLD score, FEV1/FVC, MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration subgroups were significantly different between the two groups.
This study contains the following: 1) evidence that FEV1 is significantly correlated with diaphragm movement, 2) cutoff values for diaphragm movement in patients with COPD, and 3) evidence to support the claim that the function of the diaphragm should be considered when interpreting the patient’s condition based on their FEV1.
First, FEV1 was significantly correlated with diaphragm movement. Studies on the relationship between the diaphragm and pulmonary function in patients with COPD are ongoing and have consistently reported that the severity of COPD and diaphragm function are closely related. Some previous studies have evaluated the direct relationship between FEV1 and diaphragm function [ 23 , 24 ].
The results of this study is also consistent with those of previous studies showing that diaphragm movement and FEV1 are related. However, beyond the findings of previous results [ 23 ], in our study, diaphragmatic excursion and thickness were found to be more correlated to FEV1 on the right side than on the left side.
Like the previous study that the thickness of the diaphragm is related to the ventilator weaning mechanical ventilation [ 9 , 10 ], this result has confirmed that the right diaphragm thickness was significantly related not only to the weaning of the ventilator and the prognosis of the patient but also to FEV1.
Second, we provided a cutoff value for a right diaphragmatic forced excursion in patients with COPD. Although there are studies that have presented a reference [ 23 ] value for healthy persons, the significant contribution of this study is the proposed reference value for patients with COPD.
We analyzed the correlation using Pearson’s correlation coefficient and confirmed the factors of diaphragmatic function-related components side (right, left), thickness, and excursion that were most-related to FEV1. Among them, Rt. forced excursion (supine), Lt. forced excursion (supine) and Rt. end-expiratory thickness showed meaningful p -value in association with FEV1. In addition, these three factors were analyzed in the linear relationship with the scattered plot and showed a proportional relationship between FEV1. Finally, when all factors related to the diaphragmatic function were analyzed, the right forced excursion was statistically determined as the most meaningful factor in relation to FEV1. We also obtained the cut-off value of 6.7 cm through the ROC curve.
The range in diaphragmatic excursion values varies considerably depending on the patient’s condition. A previous study has suggested normal values based on sex and the side of the diaphragm using healthy individuals. When breathing deeply, the right diaphragmatic excursion was 7 cm ± 1.1 in men and 5.7 cm ± 1 in women ( p < 0.001) and the left diaphragmatic excursion were 7.5 cm ± 0.9 and 6.4 cm ± 1 in men and women, respectively ( p < 0.01) [ 23 ]. In our study, we also assessed excursion during deep breathing to provide a cut-off value for patients with COPD.
When analyzed by dividing them into two groups based on a cut-off value, the following evaluation factors showed significant differences ( p < 0.05): FEV1, FVC, MIP, left forced excursion, right diaphragmatic thickness during end-inspiration, 6MWT, the SpO2 before and after 6MWT, and interruption of the 6MWT.
These factors can be broadly divided into PFT-related and performance-related factors. As mentioned above, PFT-related factors such as MIP, left diaphragmatic forced excursion and right diaphragmatic thickness during end-inspiration were lower in the < 6.7 cm group. Moreover, the SpO2 level before the 6MWT was lower in the < 6.7 cm group, the overall 6MWT was shorter, and there were many interruptions in the 6MWT. These factors might reflect activity as a performance evaluation factor. Although generalizability is limited given the few patients and the fact that all the participants were outpatients who could walk; these results may reflect an actual patient’s status. However, these findings are intended for patients who can walk, suggesting that the cut-off value of 6.7 cm may be reliable in this population.
Finally, results concerning the degree of pulmonary function and correlations with the diaphragmatic movement were noteworthy. The two groups were analyzed based on the right diaphragmatic forced excursion (6.7 cm) and divided into subgroups based on FEV1 (< 50% vs. ≥ 50%). As a result, in the group that had maintained diaphragm function (≥ 6.7 cm), the MIP, portable peak flow meter, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration were different between the two FEV1 groups. In summary, the difference between the two FEV1 groups was large when diaphragm function was maintained; when it was not maintained, there were no differences between the two FEV1 groups. Therefore, even in patients who maintained their FEV1 > 50%, when diaphragm function deteriorated, the patient’s 6MWT, SpO2 before and after the 6MWT were less predictable (they either deteriorated or were maintained). The patients whose FEV1 decreased < 50%, if the diaphragm function was maintained, the 6MWT could be better than that in patients with an FEV1 ≥ 50% and a reduced diaphragm function.
In conclusion, when interpreting a patient’s condition based on FEV1, it is important to assess diaphragm function, since the effect of the FEV1 value on the patient depends on how well the diaphragm function has been maintained.
In this study, when the diaphragm function was maintained, there were significant differences in MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration according to FEV1 in patients with COPD. Even if the diaphragm function was not maintained, because there are still differences in the FEV1, it may be beneficial to consider diaphragmatic function measured by right diaphragm excursion as an additional indicator of function beyond the FEV1. Therefore, it can be clinically helpful to check whether the diaphragm is functioning properly when determining a patient’s condition based on FEV1.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic obstructive pulmonary disease
Pulmonary function test
- 6-minute walk test
Forced expiratory volume in the first second
Maximal inspiratory pressure
International Classification of Diseases 11TH
Forced vital capacity
Global Initiative for Chronic Obstructive Lung Disease
Computed tomography
Maximal expiratory pressure
Bioelectrical impedance analysis
Modified Medical Research Council
Receiver operating characteristic
Body mass index
St. George's Respiratory Questionnaire
Niedermeyer J. Dyspnea in airway and pulmonary diseases. Internist. 2015;56(8):882–9.
Article CAS Google Scholar
Antoniu SA. Descriptors of dyspnea in obstructive lung diseases. Multidisciplinary respiratory medicine. 2010;5(3):216–9.
Article Google Scholar
Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412.
Decramer M. Respiratory muscles in COPD: regulation of trophical status Verhandelingen. Koninklijke Academie voor Geneeskunde van Belgie. 2001;63(6):577–602 discussion −4.
CAS Google Scholar
Corbellini C, Boussuges A, Villafañe JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63(10):1271–80.
Crimi C, Heffler E, Augelletti T, Campisi R, Noto A, Vancheri C, et al. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int J Chronic Obstruct Pulmon Dis. 2018;13:3131–9.
He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014;192(4):553–61.
Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48.
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact Inspiratory Effort Am J Respirat Cri Care Med. 2015;192(9):1080–8.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–13.
Boussuges A, Rives S, Finance J, Brégeon F. Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases. 2020;8(12):2408–24.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29.
Criner G. 6-minute walk testing in COPD: is it reproducible? Eur Respir J. 2011;38(2):244–5.
Hernandes NA, Wouters EF, Meijer K, Annegarn J, Pitta F, Spruit MA. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J. 2011;38(2):261–7.
Chen H, Liang BM, Tang YJ, Xu ZB, Wang K, Yi Q, et al. Relationship between 6-minute walk test and pulmonary function test in stable chronic obstructive pulmonary disease with different severities. Chin Med J. 2012;125(17):3053–8.
Google Scholar
Minami T, Manzoor K, McCool FD. Assessing diaphragm function in Chest Wall and neuromuscular diseases. Clin Chest Med. 2018;39(2):335–44.
ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166(4):518–624.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39(5):801–10.
Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med. 1991;85 Suppl B(25-31):discussion 3-7.
Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC pulmonary medicine. 2012;12:61.
Dhungana A, Khilnani G, Hadda V, Guleria R. Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Critical Care Med. 2017;6(4):185–9.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400.
Rocha FR, Brüggemann AK, Francisco DS, Medeiros CS, Rosal D, Paulin E. Diaphragmatic mobility: relationship with lung function, respiratory muscle strength, dyspnea, and physical activity in daily life in patients with COPD. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2017;43(1):32–7.
Download references
Acknowledgements
Abstract has been published/presented in the Korean tuberculosis and respiratory society, the Korean tuberculosis and respiratory society fall academic presentation | 129 volume 0342 ~ 343, total 2 PAGES, 2021
https://journal.kstudy.com/ISS_Detail.asp?key=3921544&tname=kiss2002&code=YqldZWtoSqVtJTNEOTEnMSUmN/B%20Z%20xLJTNEVHJpZSUmNbNj2bRU4XB/JTNEMA ==
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology (30–2020-003), Pusan National University Yangsan Hospital. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Taehwa Kim and Sungchul Huh contributed equally to this work.
Authors and Affiliations
Division of Respiratory, Allergy and Critical Care Medicine, Department of Internal Medicine, Pusan National University Yangsan Hospital and Pusan National University School of Medicine, Geumo-ro 20, Beomeo-ri, Yangsan-si, Gyeongsangnam-do, 50612, Republic of Korea
Taehwa Kim, Jae Heun Chung, Yun Seong Kim & Seung Eun Lee
BioMedical Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, South Korea
Department of Rehabilitation Medicine, Rehabilitation Hospital, Pusan National University Yangsan, Yangsan, South Korea
Sungchul Huh & Ra Yu Yun
Pusan National University School of Medicine, Yangsan, South Korea
College of Nursing, Pusan National University, Pusan National University Yangsan Hospital, Yangsan, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: TK, SEL. Data acquisition and analysis: TK, OP, RYY, SH, JHC, SEL. Data interpretation: TK, RYY, SH, JHC, SEL. Validation: TK, JHC. Writing – original draft: SH, TK. Writing – review: SEL, JHC, YSK. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Seung Eun Lee .
Ethics declarations
Ethics approval and consent to participate.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) [ 17 ]. The study was approved by Pusan National University Yangsan Hospital (PNUYH) Institutional Review Board (IRB No. 05–2020-217) and individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest or funding sources to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, T., Huh, S., Chung, J.H. et al. Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease. BMC Pulm Med 23 , 33 (2023). https://doi.org/10.1186/s12890-022-02220-7
Download citation
Received : 25 April 2022
Accepted : 02 November 2022
Published : 27 January 2023
DOI : https://doi.org/10.1186/s12890-022-02220-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cut-off value
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]

- Diaphragmatic paralysis
- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Umamaheswara Reddy V had no recorded disclosures.
At the time the article was last revised Mostafa Elfeky had no recorded disclosures.
- Paralysis of diaphragm
- Paralysis of the diaphragm
Diaphragmatic paralysis (also considered very similar to the term diaphragmatic palsy ) can be unilateral or bilateral.
On this page:
Clinical presentation, radiographic features, treatment and prognosis, differential diagnosis.
- Cases and figures
Clinical features are highly variable according to underlying etiological factors:
- may have dyspnea, headaches, fatigue, insomnia and overall breathing difficulty
- bilateral diaphragmatic palsy can be a medical emergency; they present with severe dyspnea, even with mild exertion
- idiopathic: accounts for ~70% of the cases
- bronchogenic carcinoma and other neoplasms
- spinal cord injury
- cardiac surgery 7
- Erb's palsy (birth trauma)
- myasthenia gravis
- polymyositis
- muscular dystrophies
- iatrogenic causes
- cerebral hypoventilation syndrome (Ondine's curse)
ADVERTISEMENT: Supporters see fewer/no ads
Plain radiograph
Normally the right dome of the diaphragm is higher in position as compared to the left dome, if the left dome of the diaphragm is elevated (>2 cm) diaphragmatic palsy should be suspected.
Fluoroscopy
Fluoroscopic examination of the diaphragm (" sniff test ") is very useful in diagnosing diaphragmatic paralysis. In normal individuals, both hemidiaphragm will descend with inspiration. In cases of unilateral diaphragmatic paralysis, the affected side demonstrates a paradoxical upward movement.
An alternative to fluoroscopy in diagnosing this condition, particularly useful in the pediatric population. Real-time ultrasound is ideal for evaluation of spontaneous respiratory diaphragmatic motion (may require temporary disconnection of the ventilator). This can be performed in the axial plane to compare the two hemidiaphragm simultaneously. Additional coronal or sagittal M-mode can help quantify the degree of movement of each individual hemidiaphragm. Diagnostic criteria include paradoxical movement, excursion of less than 4 mm, and a difference >50% between the excursion of one hemidiaphragm compared to the other.
Bedside ultrasound has been used in a critical care setting for the detection of diaphragmatic dysfunction with a high degree of specificity; the lower limit of normal was defined as 1 cm when observing diaphragmatic craniocaudal excursion in the mid-clavicular line 8 . The ability to apply this information and predict the success of weaning a patient from mechanical ventilation tends to be more robust when one measures the contractile nature of the diaphragmatic muscle itself. Medial angulation from a sagittal transducer position in the mid-axillary line allows visualization of the striated, mixed echogenicity band just cephalad to the liver. After placing an M-mode line, one may pause the recording and measure the end-expiratory and end-inspiratory figures, the latter of which should be larger, and calculate a diaphragmatic thickening fraction ; values above 30%, indicating no sonographic diaphragmatic dysfunction, have been found to be 71% specific for extubation success 9 .
The thickening fraction of the intercostal muscles as an index of diaphragmatic dysfunction and the use of accessory muscles has a linear, negative relationship with the calculated thickening index of the diaphragm, although insufficient evidence exists to advocate its routine use at this time. Intercostal thickening fractions >8% have, thus far, been deemed pathologic 10 .
Patients with unilateral diaphragmatic paralysis do not require treatment. There may be an option for phrenic nerve stimulation in some cases.
On a chest radiograph consider:
- right diaphragmatic eventration
- lobar collapse
- subphrenic abscess
- subdiaphragmatic mass
- diaphragmatic dysfunction
- 1. Verhey PT, Gosselin MV, Primack SL et-al. Differentiating diaphragmatic paralysis and eventration. Acad Radiol. 2007;14 (4): 420-5. doi:10.1016/j.acra.2007.01.027 - Pubmed citation
- 2. Wilcox PG, Pardy RL. Diaphragmatic weakness and paralysis. Lung. 1989;167 (6): 323-41. Pubmed citation
- 3. Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med. 2009;30 (03): 315-20. doi:10.1055/s-0029-1222445 - Pubmed citation
- 4. Laroche CM, Mier AK, Moxham J et-al. Diaphragm strength in patients with recent hemidiaphragm paralysis. Thorax. 1988;43 (3): 170-4. Free text at pubmed - Pubmed citation
- 5. Valls-Solé J, Solans M. Idiopathic bilateral diaphragmatic paralysis. Muscle Nerve. 2002;25 (4): 619-23. Pubmed citation
- 6. Harriet Paltiel. Pediatric Ultrasound, An Issue of Ultrasound Clinics,. (2013) ISBN: 9781455773701
- 7. Talwar S, Agarwala S, Mittal C, Choudhary S, Airan B. Diaphragmatic Palsy After Cardiac Surgical Procedures in Patients with Congenital Heart. Ann Pediatr Card. 2010;3(1):50. doi:10.4103/0974-2069.64370 - Pubmed
- 8. Pirompanich P, Romsaiyut S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. (2018) Journal of intensive care. 6: 6. doi:10.1186/s40560-018-0277-9 - Pubmed
- 9. DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. (2014) Thorax. 69 (5): 423-7. doi:10.1136/thoraxjnl-2013-204111 - Pubmed
- 10. Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. (2013) Intensive care medicine. 39 (5): 801-10. doi:10.1007/s00134-013-2823-1 - Pubmed
Incoming Links
- Passive atelectasis
- Diaphragmatic dysfunction
- Diaphragmatic palsy
- Phrenic nerve stimulation
- Polymyositis (pulmonary manifestations)
- Subpulmonic effusion
- Hemidiaphragmatic paralysis
- Left phrenic nerve palsy
- Bilateral pneumothoraces (capnothoraces)
- Right diaphragmatic paralysis
Promoted articles (advertising)
By section:.
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Open access
- Published: 18 April 2024
Positive effect of deep diaphragmatic breathing training on gastroesophageal reflux-induced chronic cough: a clinical randomized controlled study
- Shanshan Niu 1 , 2 na1 ,
- Tongyangzi Zhang 1 na1 ,
- Wanzhen Li 1 ,
- Siwan Wen 1 ,
- Lei Dong 1 ,
- Shengyuan Wang 1 ,
- Wenbo Shi 1 ,
- Cuiqin Shi 1 ,
- Yuqin Shen 4 ,
- Qianchun Huang 4 ,
- Yaling Tan 5 ,
- Xianghuai Xu 1 &
- Li Yu 1 , 3
Respiratory Research volume 25 , Article number: 169 ( 2024 ) Cite this article
278 Accesses
Metrics details
Background and Objective
To explore the efficacy of deep diaphragmatic breathing training (DEP) in patients with gastroesophageal reflux-induced chronic cough (GERC).
A randomized controlled study was conducted involving 60 GERC patients who were divided into the intervention group and the control group (each with 30 patients). Both groups received routine medication treatment for GERC, while the intervention group received DEP training additionally. Both groups were evaluated by cough symptom scores, Hull airway reflux questionnaire (HARQ), gastroesophageal reflux diagnostic questionnaire (GerdQ), generalized anxiety disorder scale-7 (GAD-7), patient health questionnaire-9 (PHQ-9), Pittsburgh sleep quality index (PSQI), the Leicester cough questionnaire (LCQ), as well as capsaicin cough sensitivity testing, B-ultrasound and surface electromyography (sEMG) of the diaphragmatic muscles before and after treatment. The cough resolution rate and changes of the above indictors was compared between the two groups after eight weeks of treatment.
After eight weeks of treatment, cough symptoms improved in both groups, but the cough resolution rate in the intervention group of 94% was significantly higher than that in the control group of 77% (χ 2 = 6.402, P = 0.041). The intervention group showed significant improvements to the control group in GerdQ (6.13(0.35) VS 6.57(0.77)), GAD-7 (0(0;1) VS 1(0;3)), PSQI (2(1;3) VS 4(3;6)), LCQ (17.19(1.56) VS 15.88(1.92)) and PHQ-9 (0(0;0) VS 0(0;3)) after treatment. Compared to control group, sEMG activity of the diaphragmatic muscle was significantly increased in the intervention group after treatment, measured during DEP (79.00(2.49) VS 74.65 (1.93)) and quiet breathing (72.73 (1.96) VS 67.15 (2.48)).
DEP training can improve cough symptoms as an adjunctive treatment in GERC patients.
Trial registration
The protocol was registered in February 2, 2022 via the Chinese Clinical Trials Register ( http://www.chictr.org.cn/ ) [ChiCTR2200056246].
Introduction
Gastroesophageal reflux-induced chronic cough (GERC) is a common subtype of gastroesophageal reflux disease (GERD) characterized by chronic cough as the main symptom [ 1 , 2 , 3 ]. The incidence of GERC varies by region and accounts for 5 to 40% of the causes of chronic cough [ 3 , 4 ]. With the deepening understanding, advances in examination methods and changes in lifestyles and dietary structure of GERC patients, the rate of GERC in China is increasing [ 5 , 6 ]. Current guidelines in China recommend a standard anti-reflux treatment course of at least eight weeks, but 36% of patients still require the use of neuro regulators to improve treatment, which often results in side effects such as drowsiness and dizziness [ 7 ]. The treatment of GERC, therefore, remains challenging with significant impacts on patient’s quality of life and economic prospects [ 8 , 9 ].
The main pathogenesis of GERD is the weakening of the anti-reflux barrier [ 10 ]. The high-pressure zone at the gastroesophageal junction, formed by the lower esophageal sphincter (LES), diaphragm and related structures, is a critical part of the anti-reflux barrier [ 11 ]. Once the function of the diaphragm and LES is impaired, the anti-reflux barrier weakens, leading to the occurrence of GERD.
Deep diaphragmatic breathing (DEP) training transforms chest breathing or mixed chest and abdominal breathing into DEP, using the contraction and relaxation of the diaphragm muscle to achieve deep and slow rhythmic breathing. Several recent studies have shown that DEP can improve symptoms in patients with chronic obstructive pulmonary disease by enhancing the function of the diaphragm [ 12 , 13 ]. Eherer et al. found that DEP can improve the quality of life of GERD patients, reduce esophageal acid exposure time and hypothesized that DEP could enhance diaphragmatic muscle tension to strengthen the anti-reflux barrier and improve symptoms of gastroesophageal reflux [ 14 ]. The use of DEP can also enhance the pinchcock effect of the diaphragm on the LES, strengthening the anti-reflux barrier [ 11 ]. Since GERC is a subtype of GERD and the cough symptoms in GERC patients are also partially due to impaired anti-reflux barrier function, it is hypothesized that DEP may have value as a new safe and non-invasive auxiliary treatment option in GERC treatment.
This prospective randomized controlled study aimed to explore the effects of combining DEP with anti-reflux drug therapy compared to drug therapy alone on cough symptoms, reflux symptoms, quality of life as well as sleep and psychological conditions in GERC patients.
This was a single-center, randomized, controlled prospective study that recruited suspected GERC patients who visited our department from August 2021 to December 2022. Complete medical history, physical examination, capsaicin cough sensitivity test, chest CT or X-ray examination, pulmonary function test, histamine bronchial provocation test, induced sputum cytology examination and multichannel intraluminal esophageal impedance and pH monitoring (MII-pH) data were collected. The research plan was approved by the Ethics Committee (2021-064) and registered in the Chinese Clinical Trial Registry. (ChiCTR2200056246). All study subjects were informed and signed informed consent forms.
The inclusion criteria included: ① suspected GERC, aged between 18 and 80 years, and had a cough course exceeding eight weeks; ② these patients had no obvious abnormalities on chest X-ray or chest CT images, pulmonary function with forced expiratory volume in one second/forced vital capacity (FEV1/FVC) exceeding 70%, percentage of predicted FEV1 value exceeding 80% of the expected value and ③ were able to complete DEP training. ④ MII-pH where acid exposure time (AET) exceeded 6% and/or symptom association probability (SAP) exceeding 95% and/or symptom index (SI) exceeding 50%. The exclusion criteria included: ① pregnant or lactating women, smoking or smoking cessation of fewer than two years; ② abnormal moist rales on lung auscultation; ③ symptoms such as fever, hemoptysis and dyspnea; ④ who were unable to read and understand the questionnaire, refusal to sign the informed consent form. And, the patient who had incomplete data or were violated of the treatment plan and loss of follow-up would be excluded from analysis.
The GERC diagnosis criteria [ 2 , 3 , 9 , 15 , 16 ] included a cough duration exceeding eight weeks, with or without typical reflux symptoms such as acid regurgitation and heartburn, MII-pH where acid exposure time (AET) exceeded 6% and/or symptom association probability (SAP) exceeding 95% and/or symptom index (SI) exceeding 50% and cough responsive to a stepwise anti-reflux therapy (cough symptom score decreased by > 50%).
Before the enrollment and follow-up period, both groups received health education in the out-patient department: (a) avoid oversaturated bedtime eating, acid, spicy and greasy food, coffee, acid drinks and smoking; (b) head of the bed elevation and avoiding meals within 3 hours of bedtime. After enrollment, subjects were randomly divided into the intervention and the control group by computer-generated numbers. Patients were scheduled at separate times to receive individual attention and to avoid interparticipant contact. Moreover, to reduce the chance of bias emerging, team members separately acted as participant interviewers, data collators and evaluators to ensure all data were handled objectively. The cough symptom score, capsaicin cough sensitivity, Hull airway reflux questionnaire (HARQ), gastroesophageal reflux diagnostic questionnaire (GerdQ), generalized anxiety disorder scale-7 (GAD-7), patient health questionnaire-9 (PHQ-9), Pittsburgh sleep quality index (PSQI), the Leicester cough questionnaire (LCQ) was evaluated every two weeks for eight weeks. The changes in the above observation indexes at each time point in the two groups of patients were analyzed and the cough treatment effectiveness rate and the time difference of relief of each observation index were evaluated. Before and after treatment evaluated diaphragm muscle function by, diaphragm mobility, diaphragmatic thickening fraction measured by ultrasound and surface diaphragmatic EMG activity detected by surface electromyography (sEMG) were measured to compare the differences between the two groups and further evaluate the effect of DEP on the diaphragm. The consort flow diagram of study is shown in Fig. 1 .
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of the study. ICF: inform consent form; PP: pre-protocol; GERC: Gastroesophageal reflux-induced chronic cough; HARQ: Hull airway reflux questionnaire; GerdQ: Gastroesophageal reflux disease questionnaire; PSQI: Pittsburgh sleep quality index; LCQ: Leicester cough questionnaire; GAD-7: Generalized Anxiety Disorder Scale-7; PHQ-9: Patient Health Questionnaire-9
Therapeutic regimen
Both groups were given standard anti-reflux treatment of omeprazole (AstraZeneca, China) 20 mg twice daily and mosapride (HaoSen, China) 5 mg three times daily, for eight weeks. If no remission of cough was achieved where the cough symptom score decreased by less than 50%, intensified anti-reflux treatment including increasing the dose of proton pump inhibitor (PPI) or adding a neuromodulator such as baclofen (Novartis, China) was given. In addition to this, the intervention group received professional training from a DEP rehabilitation trainer.
Briefly, when training before the study, the patient comfortably laid on the back and placed his hands on the abdomen to feel how the abdominal wall moves in and out. Repeat this exercise 5 to 10 times. Make sure that his breathing rhythm is calm and steady, and that the inflow and outflow of air feels natural. During each DEP session, the therapist tried to achieve good communication with the patient to facilitate good understanding and collaboration.
After the training, they performed independent training twice a day for 20 min each time, with a breathing frequency of six to eight breaths per minute for the eight-week trial period and specific training methods are provided in supplement 1. The patients were video-guided and were given a checklist on which they recorded whether they had undertaken training. Besides, their relations upload training videos for us. When the patient returned for a follow-up visit every two weeks, the rehabilitation trainer evaluated the patient’s progress and provided guidance for training.
Outcome measures
The primary endpoint was the rate of cough resolution, as the sum of cough control and improvement. The cough was considered to be completely controlled when it disappeared, symptom score reduction of at least 50% was considered as cough improvement, and a cough symptom score reduction of less than 50%, no improvement, or aggravation was considered ineffective.
The second end-points included the changes in capsaicin cough sensitivity, HARQ, GerdQ, GAD-7, PHQ-9, PSQI, LCQ and diaphragm muscle performance.
Auxiliary examination
For the capsaicin cough sensitivity test, based on the measurement method reported by Fujimura et al. [ 17 ], the modified method established in reference to the ERS guideline [ 18 ] was used. The minimum concentration of capsaicin required to induce > 2 (C2) or > 5 (C5) coughs as the subject’s cough threshold to evaluate the cough sensitivity to capsaicin.
The Chinese version of the cough symptom score [ 19 ], which evolved from the English version established by Hsu et al. [ 20 ] and verified clinically in the undergraduate department, was used to evaluate cough symptoms. Cough frequency and severity were divided into six levels, from zero for no cough to five for severe coughing most of the day. The Chinese version of the HARQ [ 21 ], which corresponds to the English version of the HARQ designed by Morice et al. [ 22 ], was used to assess cough hypersensitivity in patients. The GerdQ to assess reflux-related symptoms [ 23 ], was used to score reflux-related symptoms. The LCQ was used to evaluate the patient’s quality of life measure of chronic cough [ 24 ] and the PSQI was used to evaluate the patient’s sleep quality [ 25 ]. The GAD-7 [ 26 ] and PHQ-9 [ 27 ] were used to evaluate changes in patient anxiety and depressive moods.
To measure the diaphragm muscle function by ultrasound, a professional ultrasound technician used a Medison RS80A ultrasound machine (Samsung, South Korea) to measure the diaphragm mobility and thickening ratio. The measurement methods included diaphragm excursion (DE), where the subject was placed in a semi-recumbent position with the head of the bed elevated at 20 to 40° and a linear probe was placed at the intersection of the midline of the anterior chest wall and the costal arch to measure the right diaphragm through the liver as an acoustic window, scanning towards the head side. After identifying the diaphragm, the machine was switched to M-mode and the line perpendicular to the posterior one-third of the diaphragm was sampled. The distances from the baseline to the highest point during three respiratory cycles on the vertical axis were measured and averaged to obtain DE [ 28 ].
For the diaphragm thickening fraction (DTF), the patient was in the same position and the thickness of the diaphragm was measured at the intersection of the eighth to ninth intercostal space and the anterior axillary line and mid-axillary line at the end of inspiration and expiration [ 29 ]. The calculation for DTF was the difference in thickness between the end inspiration and the end of expiration divided by the thickness at the end-expiration × 100%.
Surface electrodes were used to assess EMG of the diaphragm muscle. All electromyography signals detected by the electrodes were transmitted to a biological signal acquisition and analysis system (ECH Probes, Shanghai) and amplified and band-pass filtered in the range of 5 Hz to 1 kHz, with a gain of 104 times. Under the condition of 2–6 kHz modulo sampling, the raw electromyography signals were converted into root mean square (RMS) time-domain and frequency-domain data using ECH probes electromyography acquisition and analysis software. The subjects performed a diaphragmatic maximal voluntary contraction (MVC) by performing the combined Mueller-expulsive maneuver with visual feedback and the data were normalized. The skin was lightly cleansed with alcohol to minimize electrical impedance, placing the recoding electrodes at the junction of the right sixth to eighth ribs and the anterior axillary line and the reference electrode at the bottom away from the recording electrode [ 30 ]. The electrode placement was recorded in about to 167 anatomical landmarks to ensure consistency in electrode placement between visits and as far as possible to avoid interference of intercostal muscles. The subject was placed in a semi-recumbent position with the head of the bed elevated at 20 to 40°, and observing the activity of diaphragmatic myoelectric signals to determine whether it was respiratory contraction. After the electromyography signal was free of artifacts, the sEMG of the diaphragm was continuously recorded during quiet breathing and abdominal deep breathing. From each recording 10 breaths free of artifacts were selected at the end of each period. The mean values were calculated after RMS smoothing processing and MVC normalized, respectively.
Statistical analysis
According to previous studies [ 14 ], the effect size (d) for the two-tailed test was 0.80, the alpha value (a) was 0.05 and the statistical power (1-β) was 0.80. The sample size for the two groups was one-to-one, considering a dropout rate of 10%. Using G*Power 3.197, it was calculated that each group required 29 subjects and the total sample size was 58. To study the impact of outliers on the outcome, we used the Mahalanobis distance method to analyzed the two sets of results. By applying Mahalanobis distance method the outliers were refilled using the maximum value.
The primary efficacy analysis was evaluated using the modified intention-to-treat (ITT) method, which included all patients who received at least one dose of the study medication or a training session. All efficacy analyses were also assessed using the per-protocol (PP) method. Per-protocol population criteria included the following: subject received assigned study medication and DEP, was compliant with treatment.
For normally distributed data, the mean and standard deviation (SD) was used and for skewed distributed data, the median (Q1; Q3) were used. The cough threshold values C2 and C5 are logarithmically transformed and expressed as geometric mean for categorized data. The t-test, χ 2 test, or Mann-Whitney U test were used to compare between-group and within-group differences. Statistical analysis was performed using the SPSS 24.0 software package (SPSS, USA). A P value less than 0.05 was considered statistically significant.
General information
During the study period, a total of 70 GERC patients met the inclusion criteria. Ten patients were excluded due to exclusion criteria, including four patients who refused to sign the informed consent form, two pregnant women, four other patients with incomplete data. Sixty GERC patients were enrolled in the study, with 30 patients in the intervention group (56.7% of patients required additional treatment with neuromodulators) and 30 patients in the control group (53.3% of patients required additional treatment with neuromodulators). Adherence to DEP exercise training was achieved in 29 of 34 (85.3%), and taking medication in the control group was 29 of 32(90.6%). There was no statistical difference in adherence in each group (85.3% VS 90.6%; χ 2 = 6.402, P = 0.507). There were no differences in the general clinical information and baseline observation indicators between the two groups, shown in Tables 1 , 2 and 3 . During the treatment period, one patient in the intervention group experienced persistent intolerable diarrhea after one week of treatment and refused to continue treatment. One patient in the control group did not show improvement in cough after three weeks and refused further treatment, so both were considered treatment failures.
Comparison of cough resolution rate between the two group
A total of 58 out of 60 GERC patients (97%) completed the study. After eight weeks of treatment, by the ITT analysis, the cough treatment efficacy in the intervention group of 94% was significantly higher than that in the control group at 77% (χ 2 = 6.402, P = 0.041), as same as the PP analysis (χ 2 = 7.196, P = 0.027), as shown in Fig. 2 .
Comparison of therapeutic outcomes. ( a ): the cough treatment efficacy of the training group; ( b ): the cough treatment efficacy of the control group. The rate of cough resolution in the training group is significantly higher than in the control group (94% VS 77%, P = 0.041 by ITT, P = 0.027 by PP analysis)
Comparison of scales evaluation and capsaicin cough sensitivity before and after treatment
After eight weeks of treatment, according to ITT analysis, the intervention group showed more significant improvements than the control group in terms of nighttime cough symptoms score (Z = -2.027, P = 0.043), GerdQ (t = -2.800, P = 0.007), GAD-7 (Z = -2.096, P = 0.036), PSQI (Z = -3.705, P < 0.000), LCQ (t = 2.911, P = 0.005) and PHQ-9 (Z = -2.111, P = 0.035), while there was no statistically significant difference in capsaicin cough sensitivity (C2: t = 0.685, P = 0.496; C5: t = 1.070, P = 0.289) and HARQ (t = -1.754, P = 0.085) between the two groups. The intervention group showed faster relief of the nighttime cough symptoms score than the control group in the fourth week (Z = -2.667, P = 0.007), and.
LCQ, PHQ-9 and PSQI improved faster in the sixth week than the control group, as shown in Figs. 3 and 4 .
Changes in cough symptom score from baseline to the 8-week treatment between the two groups. ( a ): changes in daytime cough symptom score over time; ( b ): changes in nighttime cough symptom score over time. In the fourth week, the training group than the control group obviously relieve nighttime cough symptoms
Changes of GerdQ, HARQ, LCQ, PSQI, GAD-7 and PHQ-9 from baseline to the 8-week treatment in the two groups. ( a ) GERC: Gastroesophageal reflux-induced chronic cough; ( b ) HARQ: Hull airway reflux questionnaire; ( c ) LCQ: Leicester cough questionnaire; ( d ) PSQI: Pittsburgh sleep quality index; ( e ) GAD-7: Generalized Anxiety Disorder Scale-7; ( f ) PHQ-9: Patient Health Questionnaire-9; ( g ) Capsaicin cough sensitivity: cough threshold C2; ( h ) Capsaicin cough sensitivity: cough threshold C5. *: P <0.05. After 8 weeks of treatment, GerdQ, LCQ, PSQI, GAD-7 and PHQ-9 in the intervention group were significantly relieved compared with those in the control group. In addition, LCQ, PSQI and PHQ-9 alleviated faster
There are also significant difference in the improvement of nighttime cough symptoms score, GerdQ, GAD-7, PSQI, LCQ and PHQ-9 in the intervention group was noted on PP analysis.
Comparison of DE, DTF and sEMGdi between the two groups
Before treatment, there was no significant difference in baseline data between the 22 patients in the intervention group and 20 in the control group, who completed the diaphragm examination ( P > 0.05) Supplementary Table 1 . The diaphragm mobility, diaphragm thickening rate and diaphragm sEMG activity of both groups during DEP were significantly higher than during quiet breathing, shown in Figs. 5 and 6 . Before treatment, the sEMGdi (training group: t = 7.808, P <0.001; control group: t = 8.172, P <0.001) during DEP has statistically significant contrast with quiet breathing which was consistent with DE (training group: t = 39.773, P <0.001; control group: t = 33.261, P <0.001) and DTF (training group: t = 17.970, P <0.001; control group: t = 14.620, P <0.001).
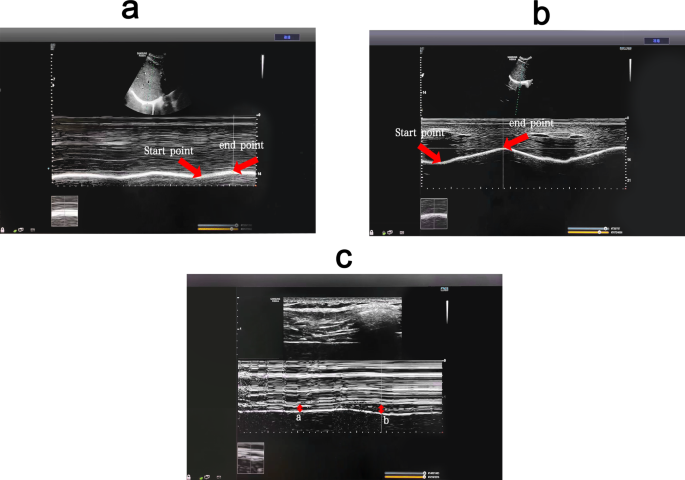
(Pre-treatment) Comparison of diaphragm excursion and diaphragm thickening fraction among breathing types. ( a ) Changes of diaphragm excursion at quiet breathing. ( b ) Changes of diaphragm excursion at abdominal deep breathing. ( c ) Changes of diaphragm thickness ( a : changes of diaphragm thickness at quiet breathing; b : changes of diaphragm thickness at abdominal deep breathing). DEP can significantly increase DE and DTF compared with quiet breathing. DEP, deep diaphragmatic breathing training; DE, diaphragmatic excursion; DTF, diaphragm thickening fraction
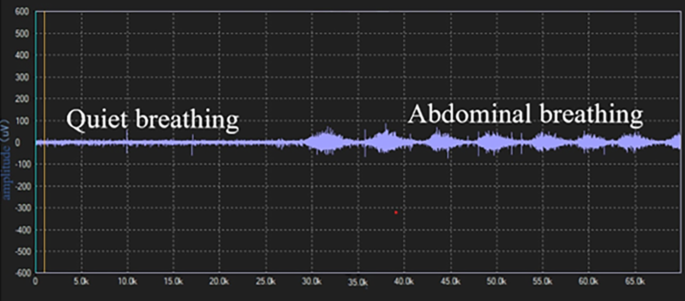
(Pre-treatment) Comparison of diaphragm EMG activity among breathing types Diaphragm sEMG activity was higher during abdominal than quiet breathing. sEMG: surface electromyogram activity
After eight weeks of treatment, the sEMGdi of the intervention group was significantly higher than that of the control group during DEP (t = 6.288, P <0.001) and quiet breathing (t = 8.136, P <0.001). The DTF of the intervention group at 169.50 (22.47) was significantly higher than that of the control group during DEP at 150.55 (25.54) (t = 2.558, P = 0.014). The measurement of DE showed that there was no statistically significant difference in diaphragm mobility between the two groups of both quiet breathing and DEP ( P > 0.05). (Table 4 .)
Comparison of DE, DTF and sEMGdi before and after treatment
In the intervention group, the post-treatment sEMG activity of the diaphragm muscle during both DEP and quiet breathing increased significantly compared to pre-treatment ( P < 0.05). The B-ultrasound measurement of diaphragm mobility during DEP of post-treatment increased significantly compared to pre-treatment( P < 0.05), while there was no statistically significant difference during quiet breathing ( P > 0.05). The DTF during both DEP and the quiet breathing of post-treatment increased compared to pre-treatment, but there was no statistically significant difference ( P > 0.05) Supplementary Table 2 . In the control group, there were no significant statistical differences observed in the post-treatment of the sEMG of the diaphragm in DEP ( P > 0.05) and quiet breathing ( P > 0.05) compared to pre-treatment. There were also no statistical differences in diaphragmatic excursion and DTF before and after treatment as well. Supplementary Table 3 .
The present study found that compared to single anti-reflux medication therapy, the combination of DEP can improve the effectiveness of GERC treatment. Compared to the control group, the intervention group showed more significant improvements in the overall evaluation of GerdQ, LCQ, PSQI, GAD-7 and PHQ-9.
The presence of GERC is an important extraesophageal manifestation of GERD. According to the pathogenesis of GERD, the weakening of the anti-reflux barrier function plays an important role in the occurrence and development of GERC. The LES and diaphragm are important components of the anti-reflux barrier. The LES is a circular muscle layer at the distal end of the esophagus. Its resting pressure is usually sufficient to prevent gastric contents from refluxing into the esophagus. However, when abdominal pressure increases, the diaphragm forms a second defense barrier to prevent reflux [ 31 ]. When LES is surgically removed, pressure can still be detected at the gastroesophageal junction [ 32 ], indicating that the diaphragm continues to maintain the anti-reflux barrier function, emphasizing the important role of the diaphragm in the anti-reflux barrier. Several studies have shown that respiratory training can increase diaphragm function [ 33 , 34 ]. The DEP technique mainly completes deep, slow and regular breathing through diaphragm contraction and relaxation. Eherer et al. found that DEP reduced acid reflux exposure in GERD patients, improved reflux symptoms and speculated that DEP training can train the crural diaphragm and reinforce the anti-reflux barrier [ 14 ].
Studies have also shown that most reflux events in GERD occur during periods of transient lower esophageal sphincter relaxation (TLESR) [ 35 ]. In addition to LES relaxation, the inhibition of the diaphragm muscle is an essential part of TLESR occurrence [ 31 ]. Banovcin et al. found that acid stimulation of the esophageal nerves can enhance gastric distension and cause a TLESR reflex, possibly by acid-activating sensory nerves in the esophagus and increasing the frequency of TLESR [ 36 ]. The use of PPIs can alleviate acid exposure-induced TLESR to some extent but cannot reduce reflux caused by LES and diaphragm dysfunction or decrease the frequency of reflux. Coughing caused by reflux is related to the total amount of proximal reflux and prolonged esophageal reflux exposure, rather than the pH value of the reflux, so most patients cannot benefit from acid suppression therapy [ 37 ].
Halland et al. found that DEP training can significantly reduce the frequency of reflux and decrease postprandial acid exposure, further improving cough symptoms in GERD [ 38 ].
Previous studies have indicated that both TLESR and the diaphragm muscle are regulated by the vagus nerve [ 39 ]. The nerve regulator baclofen is a γ-aminobutyric acid (GABA) receptor agonist that can regulate the vagus nerve pathway, reduce the occurrence of TLESR and decrease the frequency of reflux, thereby relieving cough symptoms in GERD, which is applied clinically [ 40 ]. However, some patients cannot tolerate baclofen due to the central nervous system side effects such as dizziness, drowsiness and fatigue [ 41 ]. The use of DEP training can directly or indirectly regulate the balance between sympathetic and parasympathetic nerves and is used in GERD, anxiety and other diseases [ 12 , 14 , 42 ]. Perhaps through the above mechanism, it can indirectly reduce the occurrence of TLESR, improve diaphragm function, reduce the use of baclofen and increase patient compliance with treatment.
Currently, the treatment for GERC includes medication, surgery, as well as non-pharmacological and non-surgical intervention. As people’s quality of life demands continue to rise, physical exercise and lifestyle modifications interventions for GERC are increasingly important. The guideline also points out that for suspected GERC patients without symptoms of acid reflux or heartburn, PPIs should not be the first choice and lifestyle and behavioral interventions should be prioritized [ 43 ]. Although non-pharmacological or lifestyle modifications interventions have been widely recommended for GERD patients in recent years, they are rarely mentioned for GERC patients. To the best of our knowledge, this study is the first clinical randomized controlled study on deep diaphragmatic breathing training interventions for GERC and it was concluded that this type of intervention could significantly improve the clinical symptoms of GERC patients in conjunction with medication therapy.
Based on the above mechanisms and research results, it is hypothesized that DEP training can improve the clinical symptoms of GERC patients by improving diaphragm muscle function, strengthening the anti-reflux barrier, regulating the vagal reflex, reducing the occurrence of TLESR.
To further confirm the mechanism of DEP training on the diaphragm, this study objectively evaluated diaphragm function through multiple methods. Transdiaphragmatic pressure is the main indicator for evaluating diaphragm contraction function [ 44 ], but it is invasive and difficult to widely implement in clinical practice. In recent years, studies have shown that diaphragm ultrasound can indirectly evaluate diaphragm contraction force assessing DE and DTF [ 45 ]. DE and DTF had be used to evaluate diaphragmatic function and predicted weaning from mechanical ventilation in many researches. To our knowledge, the usefulness of this technique in evaluating the changes in diaphragm function before and after DEP and speculating the effect of respiratory training on GERC has not been reported. The results showed that during DEP, diaphragm mobility was significantly increased compared to calm breathing, indicating that the diaphragm function increased accordingly, consistent with the results of Yamaguti et al. [ 13 ], and the DTF was significantly increased at post-treatment contrast to control group, indicating that DEP effectively trains the diaphragm. Compared with Wu W, et al. research on diaphragm mobility before and after rehabilitation [ 30 ], the change value did not change much and the ultrasonic sampling will be subject to echo error, for which the possibility of error cannot be excluded. The clinical significance of DEP needs to be further confirmed by large sample and multi-center independent studies. Moreover, the cause-and-effect relationship between the changes in the diaphragm and cough has not been established. Therefore, further research is necessary.
The sEMG can also quantify the work of respiratory muscles and serve as a non-invasive method to indirectly reflect respiratory muscle function [ 46 ]. In this study, sEMG was used to measure the diaphragm electromyographic activity of patients during DEP and calm breathing to evaluate changes in diaphragm contraction force. After 8 weeks of treatment, the diaphragm sEMG activity in the training group was increased in quiet breathing and deep abdominal breathing compared with those before training, in line with DE and DTF, indicating that the diaphragm function was improved under DEP. In the control group, the diaphragm electromyography activity showed an increasing trend at quiet breathing and a decreasing trend at abdominal deep breathing. The DE and DTF were not significantly or slightly increased. It may reflect that the diaphragm is prone to fatigue and its function has not improved and may be gradually deteriorating. Cough symptoms may reappear after drug withdrawal, which needs further study. The contamination of the signal picked up by surface electrodes aimed at recording diaphragm activity has also been reported. But, Similowski, et al., and Verin E, et al. [ 47 , 48 ] found that when two recording EMG electrodes are placed very close to one another, they are much more likely to record near-field potentials than far-field potentials. And the surface electrodes could be silent in response to cervical magnetic stimulation in patients with phrenic paralysis. Therefore, we believe that, surface electrodes may provide an uncontaminated diaphragm signal. And we will further to study the correlation of sEMG, di with EMGdi.
GERC is a special type of GERD manifested by a prominent cough symptom. Eherer et al. [ 14 ] research demonstrated that diaphragmatic breathing significantly reduced acid exposure and improved symptoms of GERD. Compared to the research, the patients in our study had a much wider age range, were fatter, and the standard of living was higher, leading to more difficulty in curing. Our study showed that the intervention group showed significant improvement in their gastroesophageal reflux symptoms and quality of life compared to the control group, in line with Eherer et al. research. However, the cough symptoms relief was faster than gastroesophageal reflux symptoms. Some research showed that GREC pathogenesis mainly includes two theories: reflux theory and reflex theory. DEP may not only improve diaphragmatic function, but also is significantly associated with increased thalamic GABA levels and reduced sensitivity of the cough center. The pathogenesis of GERD is complex and the prime is reflux exposure, so it is slower to relieve than cough symptoms.
In recent years, the incidence of GERC has been increasing due to changes in people’s lifestyles, improvements in corresponding diagnostic techniques and increased awareness of the disease, which is making an increasingly significant impact on people’s quality of life [ 5 ]. The LCQ, GAD-7 and PHQ-9 can measure the quality of patients’ lives. Comparing GAD-7 and PHQ-9, LCQ can comprehensively evaluate the impact of cough on patients’ lives from the physiological, psychological and social aspects. This study used the LCQ score to comprehensively evaluate changes in patients’ quality of life and found that patients who underwent DEP training were able to improve their quality of life more quickly, strengthening their treatment compliance. For chronic cough patients, especially during the pandemic, long- term uncontrollable coughing can lead to anxiety and depression, and frequent nighttime coughing can affect sleep quality, exacerbating emotional disorders. Psychological disorders can worsen patients’ sensitivity to symptoms and reduce their treatment compliance and GERD patients are more prone to comorbid anxiety and depression, leading to treatment difficulties [ 10 , 49 ] and a detrimental cycle. The DEP training is a relaxation technique that may upregulate GABA [ 50 ], regulate the balance of the sympathetic and parasympathetic nervous systems, reduce cortisol secretion, lower respiratory rate and increase heart rate variability, relieving patients’ anxiety and other emotions [ 13 , 51 ] and reducing symptom sensitivity caused by these disorders. Gu et al. found that DEP training improved patients’ psychiatric disorders and improved sleep quality by reducing negative emotions [ 42 ]. The changes in cough symptoms, anxiety and depression and sleep quality in the intervention group in this study were consistent with the above research results, further supporting the benefits of DEP training for GERC.
Gabapentin, a widely used neural regulator in clinical practice, is a GABA derivative that inhibits synaptic neurotransmitter release, thereby inhibiting the sensitivity of the cough center to reduce coughing [ 7 ]. Previous studies in this department have found that gabapentin is effective for refractory GERC, possibly because these patients have cough center hypersensitization [ 52 ] and Streeter C, et al. found that breathing was significantly associated with increased thalamic GABA levels using magnetic resonance spectroscopy [ 50 ]. , which may be another mechanism for alleviating coughing in GERC patients.
HARQ and capsaicin cough sensitivity test were related to cough hypersensitivity. In this study, the HARQ and capsaicin cough sensitivity test showed an improvement trend after 8 weeks of training while these values showed no statistically significant difference(Supplementary Table 4 ), which further confirms the DEP may inhibit the sensitivity of the cough center and relieve cough symptoms in patients with GERC.
This study had some limitations. (1) In view of the pain of the examination, patients did not want to repeat the examination, especially after the symptoms improved, so we did not require the acquisition of esophageal manometry and MII-PH data in the design of the study protocol. While the improvement in diaphragmatic muscle function was observed through B-mode ultrasound and sEMG, the changes in pressure at the gastroesophageal junction and acid exposure could not be obtained. The direct relationship between diaphragmatic muscle strength enhancement and reflux cannot therefore be confirmed. (2) The ultrasonic sampling will be subject to echo error, for which the possibility of error cannot be excluded. (3) The sample size of this study is also relatively small, mainly because the proportion of these GERC patients was very low, and it is difficult for some patients to persist in training DEP, and larger studies may be needed to support the conclusions.
Conclusions
The DEP training may increase patients’ diaphragmatic muscle function, therefore, enhance anti-reflux barriers, improve cough treatment effectiveness in patients with GERC and alleviate symptoms of gastroesophageal reflux, improve quality of life, sleep quality and alleviate anxiety and depression.
Data availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
acid exposure time
diaphragmatic excursion
deep diaphragmatic breathing training
diaphragm thickening fraction
forced expiratory volume in one second
forced vital capacity
γ-aminobutyric acid
Generalized Anxiety DisorderScale-7
gastroesophageal reflux-induced chronic cough
gastroesophageal reflux disease
Gastroesophageal reflux diagnostic questionnaire
Hull airway reflux questionnaire
inform consent form
intention-to-treat
Leicester cough questionnaire
lower esophageal sphincter
Multichannel intraluminal esophageal impedance and pH monitoring
maximal voluntary contraction
Patient Health Questionnaire-9
per-protocol
proton pump inhibitor
Pittsburgh sleep quality index
root mean square
symptom association probability
surface electromyogram activity
surface diaphragmatic EMG activity
symptom index
transient lower esophageal sphincter relaxation
Yu L, Wei W, Lv H-J, et al. Changes in the spectrum and frequency of causes for chronic cough: a retrospective analysis [J]. Chin J Tuberc Respir Dis. 2009;32:414–7.
Google Scholar
Chinese Medical Association of Respiratory Diseases Association Asthma Group. Guidelines for the diagnosis and treatment of coughing (2015). Chin J Tuberc Respir Dis. 2016;39:323–54.
Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):S80–94.
Article Google Scholar
Lai K, Chen R, Lin J et al. A prospective, multicenter survey on causes of chronic cough in China[J]. Chest, 2013, 143(3).
Ding H, Xu X, Wen S, et al. Changing etiological frequency of chronic cough in a tertiary hospital in Shanghai, China[J]. J Thorac Dis. 2019;11(8):3482–9.
Article PubMed PubMed Central Google Scholar
Chen Q, Qiu Z. Gastrointestinal Tract: Assessment and Treatment of Cough. Diagnosis and Treatment of Chronic Cough, 2021: 47–54.
Zhang M, Zhu Y, Dong R, et al. Gabapentin versus baclofen for treatment of refractory gastroesophageal reflux-induced chronic cough[J]. J Thorac Dis. 2020;12(9):5243–50.
Lv HJ, Qiu ZM. Refractory chronic cough due to gastroesophageal reflux: definition, mechanism and management[J]. World J Methodol. 2015;5(3):149–56.
Xu X, Lv H, Yu L, et al. A stepwise protocol for the treatment of refractory gastroesophageal reflux-induced chronic cough[J]. J Thorac Dis. 2016;8(1):178–85.
PubMed PubMed Central Google Scholar
Tack J, Pandolfino JE. Pathophysiology of gastroesophageal reflux Disease[J]. Gastroenterology. 2018;154(2):277–88.
Article CAS PubMed Google Scholar
Zachariah RA, Goo T, Lee RH. Mechanism and pathophysiology of gastroesophageal reflux Disease[J]. Gastrointest Endosc Clin N Am. 2020;30(2):209–26.
Article PubMed Google Scholar
Hamasaki H. Effects of diaphragmatic breathing on Health: a narrative Review[J]. Med (Basel), 2020, 7(10).
Yamaguti WP, Claudino RC, Neto AP et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil 2012;93(4).
Eherer AJ, Netolitzky F, Hogenauer C, et al. Positive effect of abdominal breathing exercise on gastroesophageal reflux disease: a randomized, controlled study[J]. Am J Gastroenterol. 2012;107(3):372–8.
Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus[J]. Gut. 2018;67(7):1351–62.
Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to anti-reflux therapy[J]. Chest. 2014;145(6):1264–70.
Fujimura M, Sakamoto S, Kamio Y, et al. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid[J]. Thorax. 1992;47(6):441–5.
Article CAS PubMed PubMed Central Google Scholar
Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–76.
Zhao T, Qiu Z, Wang L, Li Y, Lv H, Qiu Z. Validation of the reliability and clinical value of the simplified cough score. Chin J Gen Pract. 2012;11:273–6.
Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder[J]. Eur Respir J. 1994;7(7):1246–53.
Huang Y, Yu L, Xu XH, et al. Validation of the Chinese version of Hull Airway Reflux Questionnaire and its application in the evaluation of chronic cough. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39:355–61.
CAS PubMed Google Scholar
Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity[J]. Lung. 2011;189(1):73–9.
Lacy B, Chehade R, Crowell M. A prospective study to compare a Symptom-based reflux disease questionnaire and Impedance-pH monitoring for the identification of gastroesophageal reflux disease: 46[J]. Am J Gastroenterol. 2010;105:S17.
Birring SS. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ)[J]. Thorax. 2003;58(4):339.
Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis[J]. Sleep Med Rev. 2016;25:52–73.
Dear BF, Titov N, Sunderland M, et al. Psychometric comparison of the generalized anxiety disorder scale-7 and the Penn State worry questionnaire for measuring response during treatment of generalised anxiety disorder[J]. Cogn Behav Ther. 2011;40(3):216–27.
Negeri ZF, Levis B, Sun Y, et al. Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis[J]. BMJ. 2021;375:n2183.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values[J]. Chest. 2009;135(2):391–400.
Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation[J]. Crit Care Med. 2011;39(12):2627–30.
Wu W, Guan L, Li X, et al. Correlation and compatibility between surface respiratory electromyography and transesophageal diaphragmatic electromyography measurements during treadmill exercise in stable patients with COPD[J]. Int J Chron Obstruct Pulmon Dis. 2017;12:3273–80.
Boeckxstaens GE, Rohof WO. Pathophysiology of gastroesophageal reflux disease[J]. Gastroenterol Clin North Am. 2014;43(1):15–25.
Klein W. Sphincter like thoracoabdominal high pressure zone after gastrectomy[J]. Gastroenterology, 1993, 105.
Mittal RK, Rochester DF, Mccallum RW. Effect of the diaphragmatic contraction on lower-oesophageal sphincter pressure in man.[J]. Gut. 1988;28(12):1564–8.
Sun X, Shang W, Wang Z, et al. Short-term and long-term effect of diaphragm biofeedback training in gastroesophageal reflux disease: an open-label, pilot, randomized trial[J]. Dis Esophagus. 2016;29(7):829–36.
Roman S, Holloway R, Keller J et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry[J]. Neurogastroenterol Motil, 2017, 29(2).
Banovcin P Jr., Halicka J, Halickova M, et al. Studies on the regulation of transient lower esophageal sphincter relaxations (TLESRs) by acid in the esophagus and stomach[J]. Dis Esophagus. 2016;29(5):484–9.
Herregods TVK, Pauwels A, Jafari J, et al. Determinants of reflux-induced chronic cough[J]. Gut. 2017;66(12):2057–62.
Halland M, Bharucha A, Crowell M, et al. Effects of diaphragmatic breathing on the pathophysiology and treatment of upright gastroesophageal reflux: a randomized controlled Trial[J]. Am J Gastroenterol. 2021;116(1):86–94.
Mittal RK, Fisher MJ. Electrical and mechanical inhibition of the crural diaphragm during transient relaxation of the lower esophageal sphincter[J]. Gastroenterology. 1990;99(5):1265–8.
Xu X, Chen Q, Liang S, et al. Successful resolution of refractory chronic cough induced by gastroesophageal reflux with treatment of baclofen. Cough. 2012;8(1):8.
Dong R, Xu X, Yu L, et al. Randomised clinical trial: gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough[J]. Aliment Pharmacol Ther. 2019;49(6):714–22.
Fangchen GU, Meifeng WANG, Zheng LIN, et al. Effect of abdominal deep breathing exercises on gastrointestinal and psychological symptom clusters in patients with gastroesophageal reflux disease[J]. Chin J Nurs. 2019;54(4):501–5.
Kahrilas PJ, Altman KW, Chang AB, et al. Chronic Cough due to Gastroesophageal Reflux in adults: CHEST Guideline and Expert Panel Report[J]. Chest. 2016;150(6):1341–60.
Kim MJ, Druz WS, Danon J, et al. Mechanics of the canine diaphragm[J]. J Appl Physiol. 1976;41(3):369–82.
Goligher EC, Laghi F, Detsky ME, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity[J]. Intensive Care Med. 2015;41(4):642–9.
Bellani G, Mauri T, Coppadoro A, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm[J]. Crit Care Med. 2013;41(6):1483–91.
Similowski T, Mehiri S, Attali V, Duguet A, Straus C, Derenne JP. Comparison of magnetic and electrical phrenic nerve stimulation in assessment of phrenic nerve conduction time. J Appl Physiol. 1997;82:1190–9.
Verin E et al. Validation of improved recording site to measure phrenic conduction from surface electrodes in humans. J Appl Physiol 92.3(2002):967–74.
Yang XJ, Jiang HM, Hou XH, et al. Anxiety and depression in patients with gastroesophageal reflux disease and their effect on quality of life[J]. World J Gastroenterol. 2015;21(14):4302–9.
Streeter CC, Jensen JE, Perlmutter RM, et al. Yoga asana sessions increase brain GABA levels: a pilot study[J]. J Altern Complement Med. 2007;13(4):419–26.
Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy Adults[J]. Front Psychol. 2017;8:874.
Zhang Y, Qiu Z. Cough hypersensitivity syndrome [J]. Int J Respir. 2015;35(13):1015–8.
Download references

Acknowledgements
The authors are grateful to all the members of Dept. of Pulmonary and Critical Care Medicine and Tongji Hospital, Tongji University School of Medicine for the fruitful discussions and their contributions.
This study was supported by the National Natural Science Foundation of China (No. 82070102 and 82270114), the Project of Science and Technology Commission of Shanghai Municipality (No.22Y11901300, 21Y11901400 and 20ZR1451500), the Program of Shanghai Academic Research Leader (No. 22XD1422700), the Fund of Shanghai Youth Talent Support Program.
Author information
Shanshan Niua, Tongyangzi Zhanga and Wanzhen Li contributed equally to this work.
Authors and Affiliations
Department of Pulmonary and Critical Care Medicine, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, China
Shanshan Niu, Tongyangzi Zhang, Wanzhen Li, Siwan Wen, Lei Dong, Shengyuan Wang, Wenbo Shi, Cuiqin Shi, Xianghuai Xu & Li Yu
Department of Oncology, Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center), School of Medicine, Tongji University, Shanghai, China
Shanshan Niu
Department of Allergy, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, China
Department of Cardiac Rehabilitation, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, China
Yuqin Shen & Qianchun Huang
Department of Neurology, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, China
You can also search for this author in PubMed Google Scholar
Contributions
Project design: LY, XHX and SSN. Data collection: SSN, TYZZ, and WZL. Data analysis: SSN, TYZZ, WZL, SWW,SYW and WBS. Manuscript preparation: SSN, TYZZ, WZL, CQS, QCH, and YLT. Revision: LD, CQS and YQS. Approval and submission: LY, XHX, SSN, TYZZ, WZL, SWW, LD, SYW, WBS, CQS, YQS, QCH and YLT.
Corresponding authors
Correspondence to Xianghuai Xu or Li Yu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of Tongji Hospital (The ethics approval number is 2021-064). The protocol was registered in the Chinese Clinical Trials Register ( http://www.chictr.org.cn/ ) (ChiCTR2200056246). Written informed consent was obtained from all participants before enrollment.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Niu, S., Zhang, T., Li, W. et al. Positive effect of deep diaphragmatic breathing training on gastroesophageal reflux-induced chronic cough: a clinical randomized controlled study. Respir Res 25 , 169 (2024). https://doi.org/10.1186/s12931-024-02783-5
Download citation
Received : 17 October 2023
Accepted : 21 March 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s12931-024-02783-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deep diaphragmatic breathing training
- Chronic cough
- Gastroesophageal reflux
- Non-pharmacological treatment
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
- Guided tour
Moscow at Night: City Sightseeing Tour by Car/Bus
- Description
- Choose date

When the Sun begins to set, the myriad lights and firefly-like cars buzzing around on the wide highways see Moscow burst into life. This unreal sight is best enjoyed from the height of the capital’s famous viewpoints, Moscow in the evening is truly, truly spectacular. We invite you with pleasure to see the magic of Moscow under the bright night lights!
On this tour, you can expect to see incredible views and the magical transformation sunset has on Moscow’s most iconic buildings, the highlights of which being:
- The Cathedral of Christ the Saviour is a more solemn yet festive sight with its dimly lit lanterns.
- Basil's Cathedral’s fun and colourful onion domes give off the aura of a mysterious castle, growing out of the darkness of the imposing State Duma building nearby.
- The spectacularly illuminated Bolshoi Theatre and the Alexander Garden walkway, itself painted in a whimsical pattern of shadows.
- The legendary Lubyanka Square, steeped in secrecy; just its hearing its name after dark is enough to trigger goosebumps, given its murky past.
- The House on the Embankment, where old legends are revived and ghostly silhouettes lurk.
- The Russian State Library, where vague shadows have been seen flickering in its windows. Who knows, could this be the legendary bibliologist Rubakin protecting the works of literary art that lie within its walls?
- Incredible views from the height of the observation deck at Sparrow Hills. The candle-like skyscrapers of "Moscow-City" shoot into the night in a blaze of glorious light, all reflected back to the observer by the dark waters of the Moscow River. Lest not forget the blood-red fountains on Poklonnaya Hill! An absolutely fantastic spectacle which cannot be seen by day.
Our expert guides will share one thousand and one interesting stories about the aforementioned and other sights, whilst at the same time detailing the vast history of our beautiful capital, past and present.
Come with us and treat yourself to an unforgettable experience amongst the bright lights if Moscow!
The cost of an excursion with a personal guide for 1 person
Meeting point We'll pick you up at your hotel
St. Basil's Cathedral
House on the Embankment
Cathedral of Christ the Saviour
Vorobyovy Hills
Poklonnaya Hill
Moscow-City
Alexander garden
Russian State Library
Bolshoi Theatre
End of the tour
Choose your dates
Select time, who's going.
- Excursion Moscow at Night: City Sightseeing Tour by Car/Bus
- Date and time:
- Who's going:
Our 20 Best Moscow Tours of 2022
Join us on an unforgettable tour to Moscow, the capital of Russia. Imagine visiting Red Square, St. Basil’s the Kremlin and more. Moscow is one of Europe’s most vibrant cities and one of Russia’s most historical. All of our tours to Moscow are fully customizable and can be adjusted to fit any budget. Our most popular tours are listed below. Please click on the tour details to learn more or contact us for more information about our Moscow tours using the form at the side of the page. You can also schedule a call with one of our Russian travel specialists to learn more.

Classic Moscow
This is our most popular Moscow tour that includes all the most prominent sights. You will become acquainted with ancient Russia in the Kremlin, admire Russian art in the Tretyakov Gallery, listen to street musicians as you stroll along the Old Arbat street, and learn about Soviet times on the Moscow Metro tour.
Accommodation
PRIVATE TOUR

A Week in Moscow
This tour is a perfect choice for those who wish to get to know Moscow in depth. One of the highlights of this package is the KGB history tour which gives an interesting perspective on the Cold War. You will also have time for exploring the city on your own or doing extra sightseeing.

Weekend in Moscow
This tour is a great way to get acquainted with the capital of Russia if you are short of time. You will see all the main attractions of the city, the most important of which is the Kremlin - the heart of Russia. The tour starts on Friday and can be combined with a business trip.

Group Tour Moscow Break by Intourist
Russia's capital has so much to offer, from the Kremlin and the Metro to the Old Arbat street and the Tretyakov Gallery. Besides these sites, you will also visit a fascinating country estate which today is quite off the beaten path, Gorky Estate, where the Soviet leader Lenin spent the last months of his life.

Kolomenskoye Tour with transport
The history of Kolomenskoye stretches back for centuries. In 1380, Dmitri Donskoi’s army passed through Kolomenskoye on their way to the Kulikovo battlefield, and it was...
Tours by car

Kremlin, Red Sq., Cathedrals & Armory Tour
The Kremlin is truly a fascinating structure, at the same time it is an ancient tower, the city’s former military fortification, a palace, an armory, the sovereign treasury...
Walking tours

Kremlin, Red Sq., Cathedrals, Armory, Diamond Fund Tour

Old Arbat walking tour
You will be told of the street’s interesting history and view the street’s artisan culture. You will also have the opportunity to view and purchase souvenirs from the...

Tour to Sergiev Posad with transport
Considered by some to be the Russian Vatican, Sergiev Posad is the temporary residence of the Patriarch of the Russian Orthodox Church. The Trinity St. Sergius Monastery (Lavra)...

Tour to Kuskovo with transport
The Kuskovo Estate often called the Moscow Versailles due to its perfectly preserved French park, is an example of an 18th century, luxurious Moscow summer residence. Its history...

Tour to Tsaritsyno with transport
The Tsaritsyno Estate is located in the southern part of Moscow. The estate was constructed for Catherine the Great by the Russian architects Bazhenov and Kazakov in a romantic...

Moscow Metro and Old Arbat Tour
The Moscow Metro is one of the largest and most grandly built metro systems in the world. It was meant to be a showcase of the Soviet Union’s achievements for both the Russians...

Vodka Museum Tour with transport (excursion and vodka tasting)
Vodka is an important component of Russian life, an element of national identity and everyday culture. We invite you to visit the Vodka Museum and feel the atmosphere of long-gone...

Mikhail Bulgakov Apartment Museum
This apartment museum located close to Patriarch Ponds became the prototype of the "bad apartment" described in the novel "The Master and Margarita." Currently the museum's...

Kremlin, Red Sq., Cathedrals & Diamond Fund Tour

The State Museum of Lev Tolstoy Tour
Take this opportunity to learn more about the Russian writer Lev Tolstoy. During the visit to the museum you will see part of a vast collection of exhibits connected to Tolstoy...

Novodevichy Convent Tour with transport
Tour of the Novodevichy Monastery. Founded in 1524 by Grand Prince VasiliIoanovich, the original convent was enclosed by fortified walls and contained 12 towers. The structure...

City Tour with Visit to St. Basils & Red Sq. with transport
Panoramic City Tour. This Moscow tour is a great start to your trip and the best way to get acquainted with many of the city’s major highlights. Our professional guide will...

City Tour of Moscow
Head to the heart of Moscow with a professional guide on a 4-hour private walk through the city center. See Tverskaya and Old Arbat streets, Theatre Square with the world-famous...

Moscow Metro walking tour

Kremlin, Red Square and Cathedrals Tour

KGB Tour with transport
This is a very interesting and insightful tour. You will visit places connected with Stalin’s terror - a time of great repression and fear. You will be shown monuments to...

Soviet and Post-Soviet Moscow Tour
The tour begins with a drive or walk down Tverskaya Street – a Soviet masterpiece. In the years of Soviet power, Tverskaya began to undergo a transformation: it was widened...

Tretyakov State Gallery Tour
This world-famous gallery contains masterpieces of Russian art beginning in the 10th century up until today. You will view exquisite Russian icons and paintings from the 18th and...

Jewish Heritage of Moscow Tour
This tour offers a detailed look into the history and present-day life of the Jewish community of Moscow. On the tour, you will visit sites connected with the cultural and religious...

Vodka Museum Tour with transport (excursion only)

Lena, our guide in Moscow was excellent. She was very knowledgable and could answer any question we had for her. We liked that she could pick up on our interests and take us places we might not have thought of to go. When we realized that one of the places we had chosen to see would probably not be that interesting to us, she was able to arrange entry to the Diamond Fund and the Armoury for us. Riding the Metro with Lena was a real adventure and a lot of fun. In Saint Petersburg we found Anna well versed in the history of the Tsars and in the Hermitage collection. Arkady in Veliky Novgorod was a very good guide and answered all of our questions with ease. Novgorod was perhaps a long way to go for a day trip, but we did enjoy it. Vasily was a great driver to have and kept us safe with good humour and skill. We enjoyed ourselves so much, my daughter says she is already planning to return. We would both have no hesistation to recommend ExpresstoRussia to anyone we know.
Just wanted to let you know that My grandson Bruno and I couldn´t have been more pleased with our week in Moscow (6/15 - 6/21). We were absolutely enchanted with the whole experience, including getting lost a couple of times in the Metro during our free time. Although both our guides (both Eleanas) were excellent, I would particularly commend the first one (she took us to the Tatiakov, the KGB tour, and to that beautiful cemetery where so many great Russian artists, authors, composers, musicians, militarists, and politicians are buried). Her knowledge is encyclopedic; and her understanding of today´s Russia as a product of its past was, for us, truly enlightening. I will be taking another tour in Russia, with my wife, within the next two or three years. I will be in touch with you when the time comes. Meanwhile, I will refer you to other potential visitors to Russia as I meet them.
Tours to Moscow
Our Moscow tours are land only meaning that you arrange your own air travel to Russia and our expert staff meets you at the airport and handles everything else from there. Our online Airline Ticket booking system offers some of the most competitive rates to Russia available on the web so if you need tickets, please visit our Russian air ticket center . Rest assured that you will be taken great care of on one of our Moscow tours. Express to Russia has a fully staffed office in Moscow that will help to make your visit fun, informative and unforgettable. Please remember that of all these tours are private and can be adjusted to your taste. You can add, replace or skip some sights; you can add more days to the package or cut the tour short. Our specialists will be glad to help you create the tour of your dreams!

Moscow, a City Like No Other
Moscow is Russia’s largest city with a population of between 12 and 13 million. It is also Europe’s largest city and when you visit Moscow, you can feel it. The layout and architecture of the city is eclectic, ranging from crooked, ancient streets and alleyways to wide, bustling boulevards, from medieval churches to Stalin skyscrapers and to modern, glass buildings towering over everything and of course in the center of it all is the Kremlin and the magnificent Red Square. Moscow is also home to a fantastic, efficient and very beautiful metro system – each station having its own special design. In fact, Express to Russia’s Moscow metro tours and excursions are some of our most popular attractions that we offer. On our Moscow tours, you will see this and more.

Moscow Tours centering on Russian History
Moscow has a long and interesting history and has been the capital of Russia in many of its different iterations – capital of the Grand Duchy of Moscow , the Russian Empire and of course the Soviet Union (who could ever forget the Soviet Union?). Moscow, was founded in the 12th century by Prince Yuri Dolgaruki (Yuri of the long arms – he really did have long arms!). From that time on, it was home to the Russian Tsars until Peter the Great moved the capital to St. Petersburg in 1703. The city has survived invasions and sieges from the Mongols, the Tartars, the Poles, Lithuanians and Napoleon but has always persevered. Our Moscow tours will enlighten you on this great history and give you insights into Muscovites and their unique culture. Our Moscow tours show you what the city is like today but also brings to life the past. Moscow never seems to sleep and is bursting with energy. A Moscow tour with Express to Russia is truly the best way of getting to know Russia’s largest and most vibrant city.
Frequently Asked Questions From Our Travelers
What is the best time to visit moscow.
Any time of year is fine depending on what you plan to do. Summertime is pleasantly warm, ideal for exploring the city and its vibrant atmosphere, but Moscow will be much busier and accommodation is more expensive. Winter can be quite cold but beautiful nonetheless, and this is unproblematic if you intend to spend most of your trip in museums and galleries. There are also various festivals and events organised throughout the year. For more information about the best time to visit, read our guide
How many days are enough in Moscow?
If you plan your itinerary strategically and aren’t averse to a packed schedule, you can cover Moscow’s main sights over a long weekend. Most popular attractions are in the city centre, and the Moscow Metro allows you to cover much ground in a small amount of time. Ensure that your accommodation is fairly central and book tickets in advance, so that you can make the most of your days. For an informative and well-organised day out, check out our Moscow day tours with options to suit all interests.
Do they speak English in Moscow?
As Russia’s capital city, tourists are well accommodated in Moscow. There should be English-speaking staff in restaurants, bars, hotels, shops and attractions in tourist hotspots, and there are also English-speaking tourist police. Transport services have English translations on their maps and English announcements via intercom; alternatively, order taxis from the Yandex Taxi app (Russian Uber), though it’s unlikely that your taxi driver will speak English. If you get stuck and cannot communicate, it’s fine to use Google Translate.
Is it safe to travel to Moscow?
It is no less safe to travel to Moscow than to any European city if you exercise common sense and look after your belongings. As with every city some regions can be more unsavoury than others, but no tourist attractions are located there. The traffic in Moscow is notorious, so exercise caution when crossing roads. Do not take unlicensed taxis; book in advance or take public transport, which is widespread and perfectly safe. If you encounter any problems, look for the special tourist police who can help you. For more information, read our guide about staying safe in Russia .
Our travel brands include

Express to Russia
Join us on Facebook
We invite you to become a fan of our company on Facebook and read Russian news and travel stories. To become a fan, click here .
Join our own Russian Travel, Culture and Literature Club on Facebook. The club was created to be a place for everyone with an interest in Russia to get to know each other and share experiences, stories, pictures and advice. To join our club, please follow this link .
We use cookies to improve your experience on our Website, and to facilitate providing you with services available through our Website. To opt out of non-essential cookies, please click here . By continuing to use our Website, you accept our use of cookies, the terms of our Privacy Policy and Terms of Service . I agree
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
Masashi shiraishi.
1 Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka 5898511 Japan
2 Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan
Yuji Higashimoto
Ryuji sugiya, hiroki mizusawa, shuhei fujita, osamu nishiyama, shintarou kudo.
3 Inclusive Medical Science Research Institute, Morinomiya University of Medical Sciences, Osaka, Japan
Tamotsu Kimura
Yasutaka chiba.
4 Division of Biostatistics, Clinical Research Center, Kindai University School of Medicine, Osaka, Japan
Kanji Fukuda
Hisako matsumoto, associated data.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max ) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DE max . Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DE max to predict responses to PR.
Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DE max and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DE max was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DE max to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DE max .
DE max could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterised by minimally reversible airflow limitation [ 1 ]. The main feature of COPD is the inability of patients to cope with their activities of daily life due to shortness of breath. Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role [ 2 ] by increasing ventilatory workload and decreasing the pressure-generating capacity of the inspiratory muscles.
Pulmonary rehabilitation (PR) is a non-pharmacological intervention and has been reported to improve dyspnoea, exercise capacity and quality of life of patients with COPD [ 3 ]. Owing to a body of evidence, PR is now established as the standard of care for patients with COPD [ 4 ]. However, not all patients with COPD benefit from PR to the same extent. Therefore, identifying patients who are likely to achieve maximum benefit from the PR programme is crucial. So far, several studies have shown that severe airflow limitation or poor exercise tolerance at baseline may predict a better response to PR [ 5 , 6 ], but another study has reported inconsistent findings [ 7 ]. Furthermore, one study reported that patients with severe dyspnoea did not respond well to PR and patients with milder dyspnoea responded well [ 8 ].
Considering the role of DLH in the development of dyspnoea and poor exercise tolerance in patients with COPD, objective measures that reflect the degree of DLH may help in identifying good responders to PR. Previously, we reported that there was an association between increased dyspnoea due to DLH on exercise and decreased exercise capacity in patients with COPD and reduced mobility of the diaphragm, which was assessed by the maximum level of diaphragm excursion (DE max ) using ultrasonography [ 9 ]. Other research groups reported the utility of ultrasonographic assessment of diaphragmatic mobility in COPD in understanding its association with 6-min walk distance (6MWD), dyspnoea [ 10 ] and increased mortality [ 11 ].
However, there have been no reports on the association between diaphragmatic mobility and the effect of PR to improve exercise tolerance. The primary aim of this study is to clarify the role of DE max to predict the improvement in exercise tolerance after PR in patients with COPD.
Materials and methods
Study design and subjects.
This was a single-centre, observational, prospective cohort study. The study included 62 patients with clinically stable COPD who visited the Department of Respiratory Medicine and Allergology, Kindai University Hospital, between April 2018 and February 2021. The exclusion criteria included unstable medical conditions that could cause or contribute to breathlessness, such as metabolic, cardiovascular or other respiratory diseases, or any other disorders that could interfere with exercise testing, such as neuromuscular diseases or musculoskeletal problems. This study was approved by the Ethics Committee of Kindai University School of Medicine. Written informed consent was obtained from all participants.
Measurements
All participants underwent ultrasonography (Xario 200, Toshiba, Tokyo, Japan) for the assessment of their DE max . Using the liver as an acoustic window (Fig. 1 A), a convex 3.5 MHz probe was used to measure the excursions of the right hemidiaphragm according to the techniques mentioned in previous studies [ 9 , 12 , 13 ]. The M-mode cursor was rotated and placed on the axis of diaphragmatic displacement on the stored image, and displacement measurements were performed. Measurements were performed during each of the three deep breaths, and DE max was measured (Fig. 1 B). The maximum value obtained for the three deep breaths was used. 6MWD was performed to evaluate walking capacity according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) statement [ 14 – 16 ]. All participants performed the 6MWD test before and after the PR programme, and the magnitude of their perceived breathlessness and their leg fatigue was rated using a 1–10-point Borg scale. Responders to PR in exercise capacity were defined as those who demonstrated more than 30 m increase in 6MWD after the PR programme, which was the definition of minimal clinically important difference (MCID) for 6MWD [ 17 ].

Representative image of the right diaphragm. The probe was positioned below the right costal margin between the midclavicular and anterior axillary lines. A Two-dimensional ultrasonographic image of the right hemidiaphragm (B-mode). Diaphragmatic movements were recorded in M-mode during deep breathing (DE max ) ( B )
Spirometry (CHESTAC-800, Chest, Tokyo, Japan) was performed following the 2005 ATS/ERS recommendations [ 18 ] for measuring forced vital capacity (FVC), forced expiratory volume in 1 s (FEV 1 ) and inspiratory capacity. Respiratory muscle strength was assessed by measuring the maximum inspiratory pressure (PI max ) generated against an occluded airway at residual volume [ 19 ] (SP-370, Fukuda Denshi, Tokyo, Japan). A hand-held dynamometer (μTasF-1, Anima Corp., Tokyo) was used to measure quadriceps muscle strength (QMS). The impact of COPD on health status was assessed using the COPD assessment test (CAT), a patient-completed questionnaire on eight items, namely, cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy. The scores for each of the items range from 0 to 5 points, resulting in a CAT total score ranging from 0 to 40 points [ 20 ], and MCID of CAT is 2 points [ 21 ]. In all patients with COPD, emphysema was evaluated by computed tomography of the chest. A SYNAPSE VINCENT volume analyser (FUJIFILM Medical, Tokyo, Japan) was used to measure the low attenuation area (%LAA).
Rehabilitation programme
The outpatient PR programme was conducted twice a week for 12 weeks (24 sessions), including aerobic exercise training (ergometer and walking exercise) at 60–70% of peak workload for 20–40 min and upper- and lower-limb muscle strength training for 10–20 min.
Sample size
The sample size was estimated using R software. The analysis based on 6MWD data from the PR programme revealed that 40 subjects were required if the expected area under the curve (AUC) below the receiver operating characteristic (ROC) curve was 0.80, the power was 90%, and the significance level was 0.01. Furthermore, we anticipated a dropout from the PR programme. Thus, we set the sample size to 50 participants.
Statistical analysis
Responders and non-responders were compared using t -test, the Wilcoxon rank-sum test or χ 2 test, as appropriate. The paired t -test or the Wilcoxon signed-rank test was used to evaluate the changes in the parameters before and after the PR programme. The Pearson correlation coefficient was used to analyse the relationship between changes in 6MWD and independent variables because changes in 6MWD were normally distributed. Additionally, multivariate logistic regression models were used to assess the ability of variables to predict a response to PR. The ROC curve method was used to assess the ability of DE max to predict a response to PR. All statistical analyses were performed using the JMP software programme (JMP®, Version 14; SAS Institute Inc., Cary, NC, USA).
Out of the 62 patients included in the study, 50 completed the PR programme (Fig. 2 ). Two patients dropped out because of severe exacerbation of COPD, and 10 patients discontinued the PR owing to the coronavirus pandemic. Table Table1 1 presents the baseline characteristics of the participants. After the PR programme, scores for CAT, 6MWD, peak dyspnoea and leg fatigue of the modified Borg (mBorg) scale, and QMS improved significantly (Table (Table2). 2 ). Thirty patients showed an increase of > 30 m in 6MWD after PR (responders: 60%), and 20 patients (40%) were defined as non-responders. Baseline levels of %FEV 1 , 6MWD, PI max , DE max and QMS were significantly higher and those of CAT score and peak dyspnoea of mBorg scale were significantly lower in responders than in non-responders (Table (Table1). 1 ). Changes in 6MWD were significantly correlated with baseline levels of CAT, %FEV 1 , peak dyspnoea of mBorg scale, PI max , DE max (Fig. 3 ) and QMS and marginally correlated with baseline levels of 6MWD (Table (Table3 3 ).

Study flow diagram. COPD chronic obstructive pulmonary disease, PR pulmonary rehabilitation, 6MWD 6-min walk distance
Baseline characteristics of study participants
COPD chronic obstructive pulmonary disease, BMI body mass index, GOLD Global Initiative for Chronic Obstructive Lung Disease, LTOT long-term oxygen therapy, CAT COPD assessment test, FVC forced vital capacity, FEV 1 forced expiratory volume in 1 s, SpO 2 saturation of percutaneous oxygen, LAA low attenuation area, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength. Values are presented as means ± standard deviations or median (inter-quartile)
Effects of pulmonary rehabilitation (n = 50)
CAT COPD assessment test, 6MWD 6-min walk distance, mBorg modified Borg, QMS quadriceps muscle strength. Values are presented as means ± standard deviations or median (inter-quartile)

Relationship between DE max and the changes in 6MWD after pulmonary rehabilitation. Changes in 6MWD were significantly positively correlated with DE max (r = 0.72; p < 0.001). DE max maximum diaphragmatic excursion, 6MWD 6-min walk distance
Correlations between changes in 6MWD with diaphragm excursion and baseline characteristics
BMI body mass index, CAT COPD assessment test, FVC forced vital capacity, FEV 1 forced expiratory volume in 1 s, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength
In multivariate analysis, DE max alone significantly contributed to the prediction of responders (Table (Table4, 4 , Model 1). When using PI max instead of DE max because PI max and DE max showed a strong association (r = 0.73), both PI max and %FEV 1 contributed to the prediction (Table (Table4, 4 , Model 2). The area under the ROC curve of DE max to predict the responders was 0.915, with a sensitivity of 83% and a specificity of 95% at a cut-off value of 44.9 mm of DE max (Fig. 4 ). The significance of DE max in the predictability of responders remained even when the analysis was confined to severe patients (%FEV 1 < 50%, n = 23; AUC = 0.88, sensitivity = 70% and specificity = 100% at a cut-off value of 44.9 mm).
Multivariate analysis for responders to pulmonary rehabilitation
FEV 1 forced expiratory volume in 1 s, 6MWD 6-min walk distance, mBorg modified Borg, PI max maximum inspiratory pressure, DE max maximum diaphragmatic excursion, QMS quadriceps muscle strength. † Variables not included in the model

Receiver operating characteristic (ROC) curve for baseline DE max in relation to the response to pulmonary rehabilitation. ROC curve estimates the ability of DE max to predict a clinically important improvement in 6MWD (> 30 m) after pulmonary rehabilitation (AUC = 0.915, sensitivity = 83% and specificity = 95% at a cut-off point of 44.9 mm of DE max ). AUC area under the curve, 6MWD 6-min walk distance, DE max maximum diaphragmatic excursion
This is the first study to demonstrate the utility of DE max to predict the responsiveness of patients with COPD to 12-week PR. In this study, multivariate analysis revealed that greater baseline DE max was the only factor that predicted the responsiveness to PR, independent of baseline %FEV 1 . Additionally, the model using DE max had better prediction performance than that using PI max . The AUC of DE max to predict the 30 m or more improvement in 6MWD after the PR was 0.915, with a sensitivity of 83% and a specificity of 95% at 44.9 mm.
PR is beneficial to patients with chronic respiratory disease, including COPD [ 3 ], and generally improves exercise performance, health-related quality of life and dyspnoea [ 22 ], which was confirmed in this study. Ideally, PR was proven to be effective in all patients, but the response to PR varies considerably between individual patients [ 8 , 23 – 25 ]. Indeed, in this study, the improvement in 6MWD was less than that in MCID in 40% of the patients regardless of the degree of severity of COPD. Therefore, identifying predictors of a response is crucial in ensuring better PR efficacy and personalisation of PR programmes for patients with COPD.
In this study, the baseline values of %FEV 1 , PI max , DE max , QMS and 6MWD were positively associated with Δ6MWD in univariate analysis, suggesting that a better baseline condition was associated with a higher proportion of patients who achieved MCID after PR. These findings are consistent with those of previous studies that showed that patients with higher levels of %FEV 1 or FEV 1 /VC achieved greater improvement in 6MWD after PR [ 7 , 26 , 27 ] and a study in which patients with milder mMRC scores could achieve MCID of 6MWD after PR [ 8 ], but not for those with worst mMRC score, although others studies showed contradictory results [ 5 , 6 , 28 – 30 ] or found no significant baseline characteristics to predict a response to PR [ 31 ]. The discrepancy between the findings cannot be fully explained, but it might be due to the differences in the studied population and strength or length of PR. In this study, the mean %FEV 1 of the participants was 56.0%, which was relatively higher than that of other studies (mean %FEV 1 of 40–50% in most studies) [ 5 , 6 , 28 ], despite similar inclusion criteria throughout the studies, i.e., not limited to severe COPD in most studies. Thus, no ceiling effect with a PR programme that included high-intensity load exercise training for 20–40 min was observed in our population.
In this study, an important finding is that greater DE max at baseline was the only factor that predicted the responders in 6MWD after PR. In addition, the model using DE max had better prediction performance than that using PI max . The high predictability of DE max may be because of its strong association with DLH and dyspnoea during exercise, as reported previously [ 9 ]. DLH is involved in the development of dyspnoea, and both are important factors to determine the improvement in 6MWD in patients with COPD. Therefore, DE max that reflects the degree of DLH and dyspnoea during exercise was superior to other physiological indices to predict responders.
Furthermore, the virtuous cycle observed in our PR programme that included high-intensity load exercise training might be a result of the improvement in ventilation pattern. Improving the ventilation pattern would be easier with greater DE max , as shown in studies of mechanically ventilated patients [ 32 ], which may have reduced dyspnoea during exercise after 12 weeks of PR and improved exercise tolerance. Exercise therapy is a central component of PR, which significantly reduces blood lactate levels during exercise, reduces minute ventilation and improves exercise tolerance [ 33 ]. The high-intensity load exercise training, which is performed at 60–80% of the maximum oxygen uptake, has a higher physiological effect than low exercise load. Patients with greater DE max may be able to perform higher load training, which resulted in effective PR.
Diaphragm ultrasonography has been widely and successfully used to identify diaphragmatic dysfunction by showing its association with 6MWD, dyspnoea [ 10 ], extubation failure in mechanically ventilated patients [ 32 ], and increased mortality [ 11 ]. Recently, Lewinska and Shahnazzaryan proposed its use in pulmonary physiotherapy of patients with COPD [ 34 ]. In most previous studies, diaphragm ultrasonography was used to assess DE max , i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [ 35 , 36 ]. However, it is difficult to measure diaphragm thickness in patients with severe COPD because the length of the zone of apposition is shorter in patients with COPD than that in control subjects [ 37 ], whereas it is easy to measure DE max, which shows high intra- and inter-observer reliability [ 38 ]. Bhatt et al. showed that improvement in 6MWD was associated with that in DE max during forced expiration when the effectiveness of pursed lips breathing was assessed in the PR of patients with COPD [ 39 ]. Corbellini et al. demonstrated greater improvement in DE max during inspiration after PR, which was associated with an increase in the inspiratory capacity [ 40 ]. The normal and cut-off values of DE max during normal respiration, forced respiration, and voluntary sniffing have been described for each gender [ 38 ]. Thus, DE max would be a useful and reliable measure for incorporation into the PR assessment. Furthermore, in clinical settings, this objective measure of DE max has additional advantages as it requires minimum effort in patients and can be applied to the PR programme at home if portable ultrasonography is used. However, the assessment of DE max has a limitation. The procedures pertaining to positioning of patients, breathing patterns, and the selected hemidiaphragm are not standardised at present, which may hamper the routine use of DE max at this moment. Standardisation of these parameters would further facilitate the use of DE max in clinical settings and for research purpose.
There are some limitations to this study. This was a single-centre study involving a relatively small number of participants, and their baseline condition might have been relatively preserved. Nonetheless, 46% of the participants showed FEV 1 < 50%, and the utility of DE max was also observed in these patients with severe airflow limitation. Furthermore, in this study, few patients discontinued the PR programme, except for patients who discontinued during the coronavirus pandemic, which indicates that there was no severe mismatch between the PR programme and the patients’ ability to successfully complete this programme. As another limitation, we did not evaluate any malnutrition factors, which could be an important determinant of diaphragmatic mobility. Nonetheless, DE max was a stronger predictor of the effectiveness of PR than other parameters, including QMS or lung function using multivariate analysis. Further studies with a large number of patients are required, and the utility of DE max should be examined in patients with the most severe form of COPD with a low-intensity load exercise programme.
In conclusion, DE max , which is a reliable and easy to perform measurement, could adequately predict the improvement in exercise tolerance after PR in patients with COPD. Assessment of DE max could aid in making medical decisions associated with therapeutic strategies.
Acknowledgements
Not applicable.
Abbreviations
Authors’ contributions.
MS, YH, and YC made substantial contributions to the conception and design of the work. MS, YH, and RS made substantial contributions to the data acquisition. MS and HM made substantial contributions to the analysis. All of the listed authors designed the study and were involved in the interpretation of the data. MS and HM drafted the work. YH, MS, TK, YC, ON, KS, KF, YT, and HM revised the report critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
This work was supported by Grants-in-Aid for Scientific Research (21K11325).
Availability of data and materials
Declarations.
This study was approved by the Ethics Committee of Kindai University School of Medicine (31-086). Written informed consent was obtained from all participants.
If the manuscript is accepted, we approve it for publication in Respiratory Research.
The authors declare no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Drive a tank in Moscow
- Best price guarantee.
- Free transfer to/from the military ground.
- We speak your language.
- All our armored vehicles are completely safe.
- Choose us – you will not get bored – promise!
Tank driving

- Shooting AK-47

Shooting Tank main gun
Tank driving in russia.
Extreme activities
Our military vehicles

Tank tours in Moscow
“Tanks driving” is a real opportunity to variegate your everyday life, brighten up holidays or just get a bunch of emotions, a surge of adrenaline and unspeakable feel of extreme! We offer you, along with your friends, family members, colleagues or new acquaintances, to pay a visit to our tank training ground and for several hours to be in real soldier’s boots. Here you will find various supercool entertainment:
– riding in numerous types of military armoured vehicles and overcoming woodlands, swampy areas, hardly passable roads and various obstacles in a quite hardcore way;
– driving a real tank along the specifically designed routes;
– the real military world crash course, transferring you through the history of tank battles and allowing your personal participation;
– the possibility of shooting various types of small arms (machine guns, Kalashnikov rifles) while riding in armoured vehicles.
And, sure enough, all that will be accompanied by realistic sounds ambience and pyrotechnics explosions, allowing you to feel a surge of adrenaline first-hand. In addition, you will be provided by friendly attention and service, you will be able to taste the military field cuisine sitting in comfortable tents, and enjoy the real fun of military songs and dances.
You will experience an unforgettable ride in the T-80, T-72, T-34, T-14, BTR-80, BMP-1, BRM-1K and Jagdpanther.
Tank driving/riding in Moscow
What is included in the tour price:
✔️ Interpreter (English)
✔️ Military uniform
✔️ Division into teams, safety briefing, history background
✔️ Safety briefing
✔️ Tour of the exposition of military vehicles and equipment
✔️ Tank riding
✔️ Familiarization with samples of Soviet and German small arms
✔️ Lunch at the military mess.
Duration: the whole program – 1,5-2 hours. Tank riding – 30 minutes.
Tank T-34 tour price
until 01.09.2024
For a group (no more than 10 PAX)
225000 rub.
Per person from
Drive a tank yourself
Per person
The price is valid when ordering in a group on a schedule*
Tank T-55 tour price
For a group (no more than 5 PAX)
200000 rub.
Tank driving/riding — Moscow
"jagdpanther" (replica) .
What is included in the tour price:
✔️ Dressing up military uniform
✔️ Military park sightseeing tour
✔️ Tank riding (4 km through the forest)
Duration: the whole program – 1-1.5 hours. Tank riding – 15-30 minutes .
Tank tour price
until 01.09.2024
Per person
Per person (+tank rental price)
Shooting a tank gun
T-14 Main Battle Tank "ARMATA" (Replica based on the BMP-1 chassis)
✔️ Tank riding (3 km through the forest)
✔️ Field stripping and reassembling of AK-47 (master class)
✔️ Field lunch (buckwheat with stew meat, bread, sweets, tea)
✔️ Shooting Kalashnikov Assault Rifle with blank cartridges (10 shots per person)
✔️ Visit to the Museum of War Trophies
Duration: the whole program – 1.5-2 hours, tank ride – 15 minutes .
Tank tour price
For a group (no more than 6 PAX)
125000 rub.
BRDM-2, BTR-80, BMP-1, T-72, T-80
Tank tour price
BMP-1 (no more than 10 PAX)
120000 rub.
T-72 (no more than 5 PAX)
T-80 (no more than 5 PAX)
220000 rub.
PT-76 (no more than 6 PAX)
145000 rub.
✔️ Tank riding
✔️ Division into teams, safety briefing, history
✔️ Demonstration of driving capabilities of modern combat vehicles
✔️ Off-Road driving
✔️ Extreme route with slides, trenches, gullies
✔️ Obstacle course
✔️ Passage of water obstacles (only for T-72, T-80, BMP-1)
✔️ Ride over a trench – you in a trench when a tank passes over it (only for T-62, T-80, BMP-1, BTR-80)
✔️ Historical background and familiarization with samples of Soviet and Russian small arms, field stripping and reassembling of AK-47 (master class)
✔️ Training in tactical shooting techniques
Duration: the whole program – 2-2,5 hours. Tank riding – 30-45 minutes.
Additional services
Group tours
Duration: the whole program – 1.5-2 hours, tank ride – 45 minutes .
BMP-1 (per person)
Attention. The number of participants is limited. Book your tour in advance!
Best Price Guarantee
We value our customers and not prices.
If you find similar services at a lower price, inform us about the alternative proposal and we will make the best offer.
Please prove you are human by selecting the heart .
We are here to help you 7 days a week and respond within 24 hours. Plus, you can find most answers to your questions right on this page.
We organize group tours every weekend. You can join other tourists. You can also book a private tour any day and time.
Clothes: we recommend comfortable clothes of dark colour.
Shoes: you are recommended to wear a footgear resistant to dirt, dust and moisture, preferably on a thick flat sole. Do not wear shoes with heels, with a slippery sole, or ones you are afraid of spoiling. You can take a spare pair of shoes.
Accessories: hats, umbrellas, raincoats, tourist thermos – all that is quite worth of taking with you for better comfort.
By age: children from the age of four (accompanied by their parents).
By weight: passenger’s weight should not exceed 120 kg.
Our equipment is completely safe regardless of the chosen program. We also recommend you to have insurance effective in the Russian Federation.
We are always in touch
2024 tanksdriving.com

- St. Petersburg
- Snowmobiles
- Reindeer sledding
- Horse riding
- Photo with a bear
- 1-day Vladimir and Suzdal
- Patriot Park
- Monino Aviation Museum
- RKK Energia
- Moscow Metro Tour
- Forum «ARMY-2024»
- Tank biathlon
Apple introduces HomePod and HomePod mini in Malaysia

Amazing Sound

Refined Design

Elevated Experience with Multiple HomePod Speakers

Seamless Integration with the Apple Ecosystem

Smart Home Essentials
Matter Support
Customer Data Is Private Property
HomePod and the Environment
- Customers in Malaysia can order HomePod (2nd generation) for RM1,549 and HomePod mini for RM529 from apple.com/my/store , the Apple Store app, and in stores starting May 10.
- HomePod (2nd generation) is compatible with iPhone SE (2nd generation) and later, or iPhone 8 and later; or iPad Pro, iPad (5th generation) and later, iPad Air (3rd generation) and later, or iPad mini (5th generation) and later. The latest version of iOS and iPadOS is recommended.
- HomePod mini is compatible with iPhone SE, iPhone 6s or later, or iPod touch (7th generation); or iPad Pro, iPad (5th generation or later), iPad Air 2 or later, or iPad mini 4 or later. The latest version of iOS and iPadOS is recommended.
- New subscribers can get Apple Music free for six months with the purchase of any HomePod or HomePod mini.
Text of this article
29 April 2024
The lineup delivers impressive sound, enhanced Siri capabilities, and a safe and secure smart home experience
Today Apple announced HomePod (2nd generation) and HomePod mini are available in Malaysia starting May 10, bringing stunning audio experiences to every space in the home. Designed to work in perfect harmony, the new HomePod leverages advanced computational audio to deliver groundbreaking acoustics, while HomePod mini offers big sound in a beautifully compact design.
HomePod and HomePod mini users can listen to a catalogue of over 100 million songs with Apple Music; 1 enjoy room-filling audio with a single speaker, in a stereo pair, and using multiroom audio; or create a captivating home theatre experience with Apple TV 4K. With Siri, users can access a range of music knowledge, and search by artist, song, lyrics, decade, genre, mood, or activity.
Packed with innovations and Siri intelligence, both speakers provide convenient ways to manage everyday tasks and control the smart home. Users can create smart home automations using Siri, get notified when a smoke or carbon monoxide alarm is detected in their home, check temperature and humidity in a room, and use Intercom to send an announcement throughout the house — all hands-free.
HomePod delivers captivating audio quality, with rich, deep bass and stunning high frequencies, including support for immersive Spatial Audio tracks. A custom-engineered high-excursion woofer that drives the diaphragm a remarkable 20mm, built-in bass-EQ mic, and beamforming array of five tweeters around the base all work together to achieve a powerful acoustic experience. The S7 chip is combined with software and system-sensing technology to offer even more advanced computational audio that maximises the full potential of its acoustic system for a groundbreaking listening experience.
With room sensing technology, HomePod recognises sound reflections from nearby surfaces to determine if it is against a wall or free-standing, and then adapts sound in real time. Precise directional control of its beamforming array of five tweeters separates and beams direct and ambient audio, immersing listeners in crystal-clear vocals and rich instrumentation.
HomePod mini features an Apple-designed acoustic waveguide to direct the flow of sound down and out toward the bottom of the speaker for an immersive 360-degree audio experience. This allows customers to place HomePod mini almost anywhere in a room and hear consistent sound. A three-microphone array listens for “Hey Siri,” and a fourth inward-facing microphone helps isolate sound coming from the speaker to improve voice detection when music is playing.
To achieve big sound out of such a compact design, the Apple S5 chip in HomePod mini works with advanced software to analyse the unique characteristics of the music and apply complex tuning models to optimise volume, adjust the dynamic range, and control the movement of the driver and passive radiators in real time. The Apple-engineered full-range driver, powered by a neodymium magnet and a pair of force-cancelling passive radiators, enables deep bass and crisp high frequencies.
With a seamless, acoustically transparent mesh fabric and a backlit touch surface that illuminates from edge to edge, HomePod and HomePod mini boast a beautiful design that complements any space.
HomePod is available in white and midnight — a new colour made with 100 percent recycled mesh fabric — while HomePod mini comes in bold hues to add a pop of colour to the home, including orange, yellow, and blue, as well as space grey and white. Both HomePod and HomePod mini come with a colour-matched woven power cable.
Two or more HomePod or HomePod mini speakers unlock a variety of powerful features. Using multiroom audio with AirPlay, 2 users can simply say “Hey Siri,” or touch and hold the top of HomePod or HomePod mini to play the same song on multiple speakers, play different songs on different speakers, or even use them as an intercom to broadcast messages to other rooms.
Users can also create a stereo pair with two HomePod or HomePod mini speakers in the same space. 3 In addition to separating the left and right channels, a stereo pair plays each channel in perfect harmony for a truly standout listening experience.
Leveraging Ultra Wideband technology, users can hand off whatever they’re playing on iPhone — like a favourite song, podcast, or even a phone call — directly to HomePod or HomePod mini. To easily control what’s playing or receive personalised song and podcast recommendations, anyone in the home can bring an iPhone close to HomePod or HomePod mini and suggestions will surface automatically. The HomePod lineup can also recognise up to six voices, so each member of the home can hear their personal playlists, ask for reminders, and set calendar events.
The HomePod lineup easily pairs with Apple TV 4K for a powerful home theatre experience, and eARC (Enhanced Audio Return Channel) 4 support on Apple TV 4K enables customers to make HomePod or HomePod mini the audio system for all devices connected to the TV. When paired with HomePod (2nd generation) or HomePod mini, Enhance Dialogue lets users more clearly hear what is being said over the effects, action, and music in a movie or TV show on Apple TV 4K by separating the dialogue from the background noise and bringing it forward to the centre channel. Plus, with Siri on HomePod and HomePod mini, users can control what’s playing on their Apple TV 4K hands-free.
Find My on HomePod and HomePod mini makes it possible for users to locate their Apple devices, like an iPhone, by playing a sound on the misplaced device. Using Siri, users can also ask for the location of friends or loved ones who share their location via the app.
With Sound Recognition, 5 the HomePod lineup can listen for smoke and carbon monoxide alarms, and send a notification directly to the user’s iPhone if a sound is identified. The new built-in temperature and humidity sensor 6 can measure indoor environments, so users can create automations that close the blinds or turn on the fan automatically when a certain temperature is reached in a room. 7
By activating Siri, customers can control a single device or create scenes like “Good Morning” that put multiple smart home accessories to work at the same time, or set up recurring automations hands-free like “Hey Siri, open the blinds every day at 6 a.m.” A new confirmation tone indicates when a Siri request is made to control an accessory that may not visibly show a change, like a heater, or for accessories located in a different room. Ambient sounds — like ocean, forest, and rain — have also been remastered and are more integrated into the experience, enabling customers to add new sounds to scenes, automations, and alarms.
Users can also intuitively navigate, view, and organise accessories with the Home app, which offers categories for climate, lights, and security; enables easy setup and control of the smart home; includes a beautiful multicamera view; and allows users to view up to 30 days of activity history across door locks, garage doors, alarm systems, and contact sensors. Two popular HomeKit lock features — tap to unlock and PIN codes — are now available for Matter-compatible locks, providing even more ways to connect the home.
Matter enables smart home products to work across ecosystems while maintaining the highest levels of security. Apple is a member of the Connectivity Standards Alliance, which maintains the Matter standard, along with other industry leaders. HomePod and HomePod mini connect to and control Matter-enabled accessories, and serve as essential home hubs, giving users access to their smart home accessories when away from home.
Protecting customer privacy is one of Apple’s core values. All smart home communications are always end-to-end encrypted so they can’t be read by Apple, including camera recordings with HomeKit Secure Video. When Siri is used, the audio of the request is not stored by default. These features give users peace of mind that their privacy is protected at home.
HomePod and HomePod mini were designed with the environment in mind. They include 100 percent recycled rare earth elements in the speaker magnets and recycled plastic in the mesh fabric — including 100 percent recycled plastic in HomePod’s new midnight fabric. HomePod also features 100 percent recycled gold in the plating of multiple printed circuit boards. All of the wood fibres in the packaging come from responsible and recycled sources. Both speakers are free of mercury, brominated flame retardants, PVC, and beryllium.
HomePod and HomePod mini use power-efficient components and software that intelligently power the devices down during periods of inactivity. For example, through optimised power management features and a high-efficiency power supply, HomePod and HomePod mini have been designed to be efficient in their low-power mode, where the majority of their time is spent. The result is HomePod and HomePod mini are energy efficient right out of the box and consume around 75 percent less energy than the most stringent requirements for ENERGY STAR. Today, Apple is carbon neutral for global corporate operations, and by 2030, plans to be 100 percent carbon neutral across the entire manufacturing supply chain and the life cycle of every product. This means that every Apple device sold — including HomePod and HomePod mini — will have net-zero climate impact.
Pricing and Availability
- Apple Music requires a subscription.
- Multiroom audio requires multiple HomePod speakers or AirPlay-compatible speakers with the latest AirPlay software.
- Creating a HomePod stereo pair requires two of the same model HomePod speakers, such as two HomePod mini, two HomePod (2nd generation), or two HomePod (1st generation).
- Home theatre with eARC support requires Apple TV 4K (2nd generation) or later, running the latest tvOS software.
- Sound Recognition should not be relied upon in circumstances where the user may be harmed or injured, or in high-risk or emergency situations. Sound Recognition requires the updated Home architecture, which is available as a separate update in the Home app. It requires all Apple devices that access the home to be using the latest software.
- Temperature and humidity sensing is optimised for indoor, domestic settings, when ambient temperatures are around 15º C to 30º C and relative humidity is around 30 percent to 70 percent. Accuracy may decrease in some situations where audio is playing for an extended period of time at high volume levels. HomePod requires some time to calibrate the sensors immediately after starting up before results are displayed.
- Smart home accessories are sold separately.
Press Contacts
Kimberly Mah
(65) 9817 0876
Brett Galvin
(65) 9649 7784
Images in this article

IMAGES
VIDEO
COMMENTS
Diaphragmatic excursion is the movement of the thoracic diaphragm during breathing. ... Repeat on the other side, is usually higher up on the right side. If it is less than 3-5 cm the patient may have a pneumonia or a pneumothorax in which a chest x-ray is diagnostic for either. References
Right diaphragmatic excursion was shown to be significantly better in men than in women (Table 3). The same results were reported by Kantarci et al. who in their study reported a significant difference in diaphragmatic motion between male and female subjects . In their study, sex was the most significant factor affecting diaphragmatic function.
In right hemiplegic patients, Jung et al have reported that diaphragmatic excursions could be reduced on both sides. Consequently, the study of diaphragmatic motion after stroke can be of benefit in guiding medical therapy and respiratory physiotherapy and therefore, in preventing pulmonary complications.
We report that diaphragmatic excursion can perfectly predict successful weaning in patients with COVID-19. The diaphragm is the key respiratory muscle, which is responsible for ≈ 70% of the tidal volume during inspiration . Diaphragmatic dysfunction is common in critically ill patients and a pivotal factor in failure of weaning from MV.
The diaphragm is the dome-shaped structure that separates the thoracic and abdominal cavities. ... Decreased diaphragmatic excursion may be detected by percussion of the lower rib cage at end ...
The diaphragm is the primary muscle of ventilation. Dysfunction of the diaphragm is an underappreciated cause of respiratory difficulties and may be due to a wide variety of entities, including surgery, trauma, tumor, and infection. Diaphragmatic disease usually manifests as elevation at chest radiography. Functional imaging with fluoroscopy (or ultrasonography or magnetic resonance imaging ...
The receiver-operating characteristic curves showed that excursion of the right and left hemidiaphragm has the highest significant accuracy in predicting successful extubation of preterm infants among all diaphragmatic parameters (AUC is 0.98 and 0.96, respectively; p value < 0.001 for both).
Diaphragmatic excursion (DE) was calculated as the difference between axial slices through the lungs on inspiration and expiration, using the first slice at which the lung apex was evident as the cranial bound, and the first slice at which the diaphragm appeared as the caudal bound. ... [Right diaphragmatic kinetics measured by TM-mode ...
On the other hand, ultrasonographic assessment of excursions of the right diaphragm shows high intra- and interobserver reliability . Reduced movements of the diaphragm are a major risk factor for increased mortality in patients with COPD . However, the relationship between diaphragmatic mobility and DLH remains unclear in patients with COPD.
Diaphragmatic excursion (DE) was calculated as the difference between axial slices through the lungs on inspiration and expiration, using the first slice at which the lung apex was evident as the cranial bound, and the first slice at which the diaphragm appeared as the caudal bound. ... Excursion-volume relation of the right hemidiaphragm ...
the diaphragm relaxes during expiration: moves upwards. both hemidiaphragms move together. in healthy patients 1-2.5 cm of excursion is normal in quiet breathing 2. 3.6-9.2 cm of excursion is normal in deep breathing 2. up to 9 cm can be seen in young or athletic individuals in deep inspiration 2. excursion in women is slightly less than men 2
The ratio of right to left diaphragmatic excursion during quiet breathing was (1.009±0.19); maximum 181% and minimum 28%. Only 19 cases showed a right to left ratio of less than 50% (5 men and 14 women). The diaphragmatic excursion was higher in males than females. There was a significant difference in diaphragmatic excursion among age groups.
Diaphragmatic excursion and lung function were analyzed by an independent t-test. To analyze the relationship between lung function and diaphragmatic excursion, Karl Pearson's correlation coefficient test was used. ... Another limitation is that only right hemidiaphragm was assessed on ultrasonography. Further studies with larger number of ...
The right diaphragmatic forced excursion was closely related to FEV1, and analysis according to the right diaphragmatic forced excursion-based cut-off value showed a significant difference between both groups. When the diaphragm function was maintained, there was a lot of difference in the 6MWT's factors according to the FEV1 value.
In patients with normal diaphragm function, the dome of the right diaphragm projects over the anterior 5th to 6th rib and the posterior 10th rib. The left hemidiaphragm is naturally lower than the right by one interspace on average (Fig. 1A ). 5. ... Normal diaphragm excursion is between 3 and 5 cm.
DB. Consequently, right diaphragmatic motion could be measured in 19.5 subjects (140 men, 55 women). Mean excursion was 6.6 ± 1.3 ern. Left diaphragmatic excursion was obtained in only 45 volunteers (38 men, 7 women) during DB (mean, 7.3 ± 1 cm). For all the maneuvers studied, the diaphragmatic excursion was greater in men than in women. Con-
Normally the right dome of the diaphragm is higher in position as compared to the left dome, if the left dome of the diaphragm is elevated (>2 cm) diaphragmatic palsy should be suspected. ... Diagnostic criteria include paradoxical movement, excursion of less than 4 mm, and a difference >50% between the excursion of one hemidiaphragm compared ...
The measurement methods included diaphragm excursion (DE), where the subject was placed in a semi-recumbent position with the head of the bed elevated at 20 to 40° and a linear probe was placed at the intersection of the midline of the anterior chest wall and the costal arch to measure the right diaphragm through the liver as an acoustic ...
The maximum diaphragmatic excursion can be measured as the difference between the position of the diaphragm at functional residual capacity (FRC) and total lung capacity (TLC), while tidal excursion is the difference between the positional FRC and the end-inspiratory position during resting breathing. ... Although right hemidiaphragm excursion ...
Excursions are privately run and can be booked for 1 traveler all the way up to large groups of travelers. We run our Moscow city tours in every season. This is because travel to Moscow is excellent in any season. In the summer take a stroll through Gorky Park or take a riverboat tour along the Moskva River. In the winter, see Moscow's winter ...
Book. Guided tour. 3 hours. Популярные , Архитектурно-исторические. Code: 10072. When the Sun begins to set, the myriad lights and firefly-like cars buzzing around on the wide highways see Moscow burst into life. This unreal sight is best enjoyed from the height of the capital's famous viewpoints, Moscow in ...
This tour is a perfect choice for those who wish to get to know Moscow in depth. One of the highlights of this package is the KGB history tour which gives an interesting perspective on the Cold War. You will also have time for exploring the city on your own or doing extra sightseeing. $ 941 From/Per person. Details.
In most previous studies, diaphragm ultrasonography was used to assess DE max, i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [35, 36].
Welcome to Tanks Driving official website. We offer the Tank Driving and shooting experience with Moscow city shooting sports club. Contact us +79252336380
A custom-engineered high-excursion woofer that drives the diaphragm a remarkable 20mm, built-in bass-EQ mic, and beamforming array of five tweeters around the base all work together to achieve a powerful acoustic experience. ... The result is HomePod and HomePod mini are energy efficient right out of the box and consume around 75 percent less ...